Question: please solve the problem using material balance with chemical reaction, recirculation and purge, use the diagram To produce H2SO4, a process like the one shown
please solve the problem using material balance with chemical reaction, recirculation and purge, use the diagram
To produce H2SO4, a process like the one shown in the following figure is used:
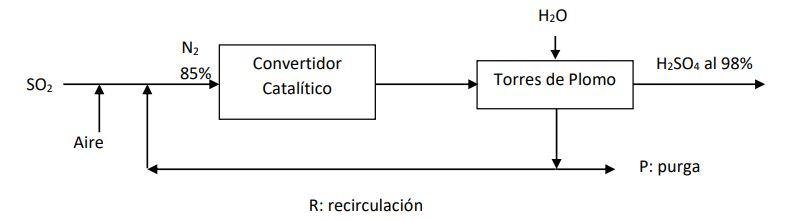
Air is fed to the process to provide the stoichiometric amount of O2 to react with SO2 to form SO3 according to the following reaction: SO2 + 1/2 O2---- SO3 In the catalytic converter said reaction is carried out with a conversion of 10%. in the towers of lead, all SO3 is dissolved in water and the remaining gases are recirculated to the converter for better utilization of SO2. However, it is necessary to purge to avoid Nitrogen accumulation. because its concentration should not be greater than 85% at the input of the converter. for a production of 10000 Kg/day of H2SO4 at 98%, find: i) The amount of water and SO2 needed ii) The quantity and composition of the gases that must be purged, and of the gases that are recirculated iii) The global conversion of the process with recirculation
Note: The exercise is complete, there is no missing information, please work with the data and information provided. can be supported by the book: Basic Principles and Calculations in Chemical Engineering-Himmelblau You can also consult the book: Elementary principles of chemical processes- Felder
H2O 1 N2 H2SO4 al 98% 85% SO2 Convertidor Cataltico Torres de Plomo Aire P: purga R: recirculacin
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


