Question: solve this problem using MATLAB and send MATLAB file at na707457@ g mail . com The Leonard-Jones potential describes the potential energy of Interaction of

solve this problem using MATLAB and send MATLAB file at na707457@ g mail . com
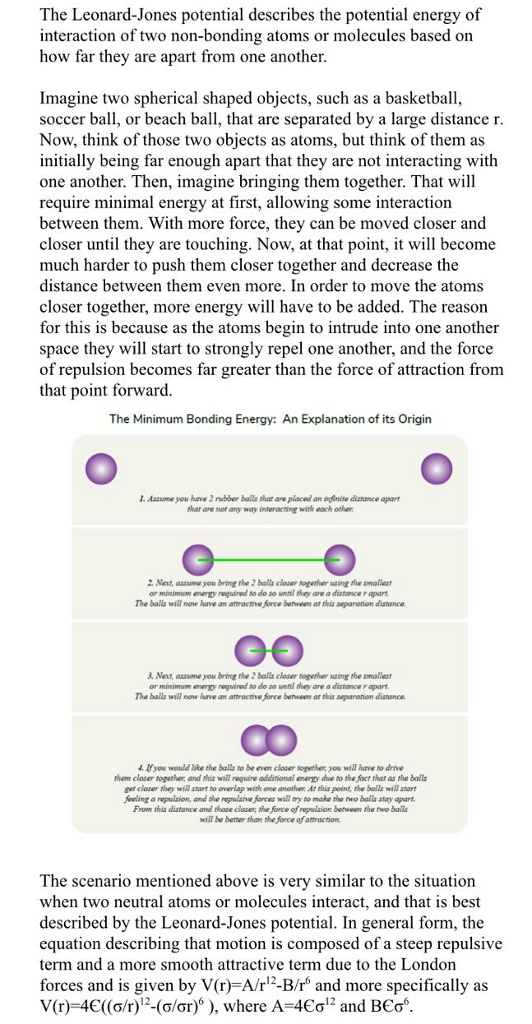
The Leonard-Jones potential describes the potential energy of Interaction of two non-bonding atoms or molecules based on how far they are apart from one another Imagine two spherical shaped objects, such as a basketball, soccer ball, or beach ball, that are separated by a large distance r Now, think of those two objects as atoms, but think of them as initially being far enough apart that they are not interacting with one another. Then, imagine bringing them together. That will require minimal energy at first, allowing some interaction between them. With more force, they can be moved closer and closer until they are touching. Now, at that point, it will become much harder to push them closer together and decrease the distance between them even more. In order to move the atoms closer together, more energy will have to be added. The reason for this is because as the atoms begin to intrude into one another space they will start to strongly repel one another, and the force of repulsion becomes far greater than the force of attraction from that point forward. The Minimum Bonding Energy: An Explanation of its Origin I. Assume you have 2 rubber bails that are placed an ingfnite distance apar are not any way interacting with each other 2. Net, asume you bring the 2 balls closer together saing the amalest or minimm enery regsared to do so unni they are a distance r apart The bails will now have an atractive force between at thia separation distance &. Next, assome you bring the 2 balls closer together asing the amallest or minimum energy reqgined to do so unni they are a distance r apart The balls will none have an atractive force berween at his separanion distance 0O If you would lake the balls to be even closer sopether, you will have to drive them closer together and this will regire addinional energy due to the fact that as the balls get closer they will start to overlap with one another Ar tiis point, the balls wall start Joeling a repulsion, and the repulainve forees will try to make the trwo baills stay apart From this distance and those clozer, the force of repuision between the two ball will be bener than the force of attaction The scenario mentioned above is very similar to the situation when two neutral atoms or molecules interact, and that is best described by the Leonard-Jones potential. In general form, the equation describing that motion is composed of a steep repulsive term and a more smooth attractive term due to the London forces and is given by V(r)-A/r12-B/r and more specifically as V(r)-4(o/2-a/or)), where A-4o1 and BEo. . Next, assume you bring the 2 bals closer together uing the smallest or minimum energy repared to do..t they arv distance rapart. The ballz will now have ' attractrveforce between at this separat on distance. GO Next, assume you brmg the 2 balls closer together usang the smallest r minimmy eguired to do so nthil they are a distance r apart The balls will now have an atractive force between at this separation distance CO 4. If you would luke the balls to be even closer together, you will have to drive them closer together, and this will require additional energy due to the fact that s tha balls get closer they will start to overlap with one another At is point, the balls will start Jeeling a nepulsion, and the repulsive forces wall try to make the two balls stay apart From this distance and those closer the force of repulsion betwem the two balls will be better than the force of attraction The scenario mentioned above is very similar to the situation when two neutral atoms or molecules interact, and that is best described by the Leonard-Jones potential. In general form, the equation describing that motion is composed of a steep repulsive term and a more smooth attractive term due to the London forces and is given by V(r)-A/r1-B/r and more specifically as V(r)-4C((o/r)12-()''), where A-4Co12 and BCo6 Remember V(r) is the intermolecular potential between the two atoms or molecules is the well depth and a measure of how strongly the two particles interact with one another. is the distance at which the intermolecular potential is zero. It is a measure of how close two non-bonding particles can get and is referred to as the Van Der Waals radius. It is always equal to /2 of the internuclear distance between to non-bonding particles r is the distance between the particles measured from the center of one atom to the center of the other Problem :For Xenon, the values for and are found 1.77 kJ/mol and 4.10 Angstroms. Using these values, plot the potential energy function for Xenon using MATLAB and approximate the radius for the lowest value of V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


