Question: solve this quesion clearly FIND A RATE EQUATION USING THE INTEGRAL METHOD Reactant A decomposes in a batch reactor Aproducts 3.t Consreent-Volume Bash Rnactor 61

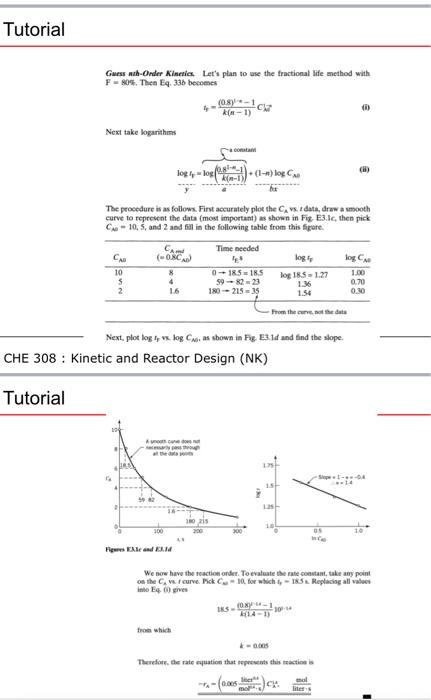
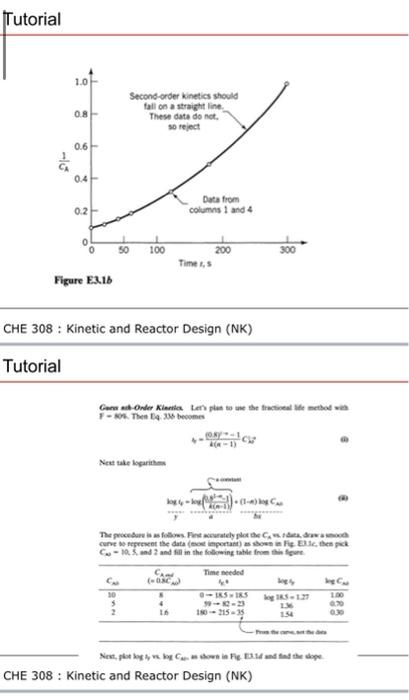
FIND A RATE EQUATION USING THE INTEGRAL METHOD Reactant A decomposes in a batch reactor Aproducts 3.t Consreent-Volume Bash Rnactor 61 The composition of A in the reactor is measared at various times with results shown in the following colamns 1 and 2. Find a rate equation to represent the data. CHE 308 : Kinetic and Reactor Design (NK) Tutorial Guess First-Order Kineties. Start by guessing the simplest rate form, or firstorder kinetics. This means that In CA0/CA vs. t should give a straight line, see Eq. 11 or 12, or Fig. 3.1. So column 3 is calculated and the plot of Fig. E3.la is made. Unfortunately, this does not give a straight line, so first-order kinetics cannot reasonably represent the data, and we must guess another rate form. CHE 308 : Kinetic and Reactor Design (NK) Guess mah-Order Kincuies Let's plan to use the fractional life method with F=80%, Thea Eq. 336 becomes t4=k(n1)(08)i1C(k Next take logarithms logry=yloonatam(k(n1)2s1x1)+(1n)logCND The proecdure is as follows. Firt accurately plot the CA, t dath, draw a sooth curve to represent the data (most important) as shown in Fiz. E3.le, then pick CA=10,5, and 2 and fill in the following table from this figare. Next, plot log Iy,x,logCAG as shown in F.e. E3. Id and find the slope. CHE 308 : Kinetic and Reactor Design (NK) Tutorial We now have the reaction order. To evaluate the nate constant, take any point into Eq. 0 gives 1.5=4(141)(0x)(41104 fron whist x=coss Terefote, the rate equation that mepvesents this maction is Gane ab-onder Kierila Lert piat to une the frectional life metbod wial I - AOl. Then La. 3M beoomes +=d(a1)6ky+1C Net tale locaritems C=10,5, and 2 and fill in the following table from the flgort. CHE 308 : Kinetic and Reactor Design (NK) FIND A RATE EQUATION USING THE INTEGRAL METHOD Reactant A decomposes in a batch reactor Aproducts 3.t Consreent-Volume Bash Rnactor 61 The composition of A in the reactor is measared at various times with results shown in the following colamns 1 and 2. Find a rate equation to represent the data. CHE 308 : Kinetic and Reactor Design (NK) Tutorial Guess First-Order Kineties. Start by guessing the simplest rate form, or firstorder kinetics. This means that In CA0/CA vs. t should give a straight line, see Eq. 11 or 12, or Fig. 3.1. So column 3 is calculated and the plot of Fig. E3.la is made. Unfortunately, this does not give a straight line, so first-order kinetics cannot reasonably represent the data, and we must guess another rate form. CHE 308 : Kinetic and Reactor Design (NK) Guess mah-Order Kincuies Let's plan to use the fractional life method with F=80%, Thea Eq. 336 becomes t4=k(n1)(08)i1C(k Next take logarithms logry=yloonatam(k(n1)2s1x1)+(1n)logCND The proecdure is as follows. Firt accurately plot the CA, t dath, draw a sooth curve to represent the data (most important) as shown in Fiz. E3.le, then pick CA=10,5, and 2 and fill in the following table from this figare. Next, plot log Iy,x,logCAG as shown in F.e. E3. Id and find the slope. CHE 308 : Kinetic and Reactor Design (NK) Tutorial We now have the reaction order. To evaluate the nate constant, take any point into Eq. 0 gives 1.5=4(141)(0x)(41104 fron whist x=coss Terefote, the rate equation that mepvesents this maction is Gane ab-onder Kierila Lert piat to une the frectional life metbod wial I - AOl. Then La. 3M beoomes +=d(a1)6ky+1C Net tale locaritems C=10,5, and 2 and fill in the following table from the flgort. CHE 308 : Kinetic and Reactor Design (NK)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


