Question: solve this question accurately. if any data is missing assume but mention it. also show all steps i will rate u 2. Suppose the order
solve this question accurately. if any data is missing assume but mention it. also show all steps i will rate u
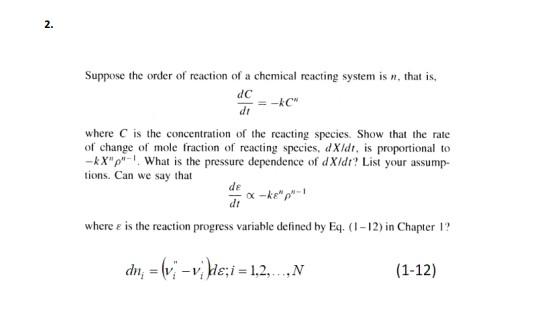
2. Suppose the order of reaction of a chemical reacting system is n, that is, dC -AC" d1 where is the concentration of the reacting species. Show that the rate of change of mole fraction of reacting species, dX/dt, is proportional to -kX"p"What is the pressure dependence of dx/dr? List your assump- tions. Can we say that Ox-ke-1 de di where e is the reaction progress variable defined by Eq. (1-12) in Chapter 1? dn, = (v;-1; }e,i = 1,2, N (1-12)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


