Question: solve using polymath with clear steps of the program , show the input/outputs and the results . P10-11 Cyclohexanol was passed over a catalyst to
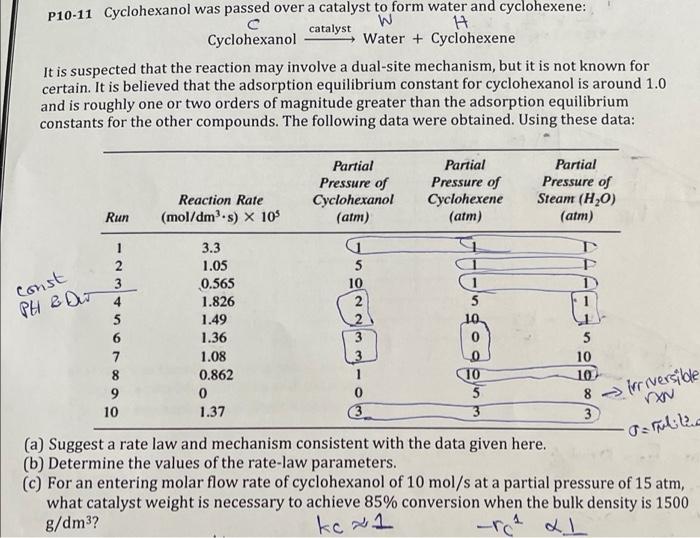
P10-11 Cyclohexanol was passed over a catalyst to form water and cyclohexene: W catalyst Cyclohexanol Water + Cyclohexene It is suspected that the reaction may involve a dual-site mechanism, but it is not known for certain. It is believed that the adsorption equilibrium constant for cyclohexanol is around 1.0 and is roughly one or two orders of magnitude greater than the adsorption equilibrium constants for the other compounds. The following data were obtained. Using these data: Partial Pressure of Cyclohexanol (atm) Partial Pressure of Cyclohexene (atm) Partial Pressure of Steam (H20) (atm) Reaction Rate (mol/dms) X 10% Run const PH & Du 3 1 3.3 T 2 1.05 5 i 3 0.565 10 1 4 1.826 2 5 1.49 ( 6 1.36 5 7 1.08 10 8 0.862 10 10 9 0 0 5 10 1.37 3 3 3 (a) Suggest a rate law and mechanism consistent with the data given here. (b) Determine the values of the rate-law parameters. (c) For an entering molar flow rate of cyclohexanol of 10 mol/s at a partial pressure of 15 atm, what catalyst weight is necessary to achieve 85% conversion when the bulk density is 1500 g/dm3? kc x1 8 irreversible Tarullia
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


