Question: solve this question using polymath plz include all the charts and plots too . P10-11 Cyclohexanol was passed over a catalyst to form water and
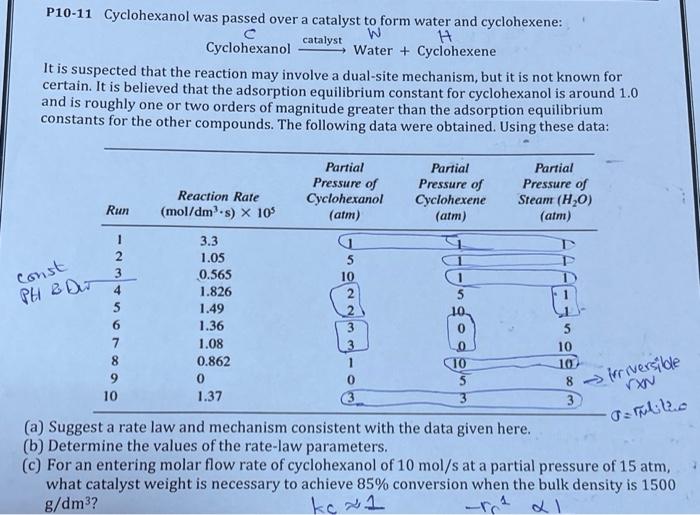
P10-11 Cyclohexanol was passed over a catalyst to form water and cyclohexene: catalyst W H Cyclohexanol Water + Cyclohexene It is suspected that the reaction may involve a dual-site mechanism, but it is not known for certain. It is believed that the adsorption equilibrium constant for cyclohexanol is around 1.0 and is roughly one or two orders of magnitude greater than the adsorption equilibrium constants for the other compounds. The following data were obtained. Using these data: Reaction Rate (mol/dms) X 105 Partial Pressure of Cyclohexanol (atm) Partial Pressure of Cyclohexene (atm) Partial Pressure of Steam (H20) (atm) Run const PH & Dur AAW 1 2 3 4 5 6 7 8 9 10 3.3 1.05 0.565 1.826 1.49 1.36 1.08 0.862 0 1.37 3 5 10 10 TO 5 8 -> irriversible x Serulito 3 (a) Suggest a rate law and mechanism consistent with the data given here. (b) Determine the values of the rate-law parameters. (C) For an entering molar flow rate of cyclohexanol of 10 mol/s at a partial pressure of 15 atm, what catalyst weight is necessary to achieve 85% conversion when the bulk density is 1500 g/dm3? -re al ke x1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


