Question: Solving For A Reactant Using A Chemical Equation Ammonlum Phosphute (H),PO, Ls An Important Ingredient In Many Fertliers It An Be Made By Reacting Phosphoric
Solving For A Reactant Using A Chemical Equation Ammonlum Phosphute (H),PO, Ls An Important Ingredient In Many Fertliers It An Be Made By Reacting Phosphoric Acd (H,PO,) With Anmenia Um Phosphate (NH Is An Important Ingredient In Many Fertilizers. T Can Be Made By Reacting Phosphoric Acid (H,POs) With Ammonia (NH,) What Mass Of Ammonium Phosphate Is Produced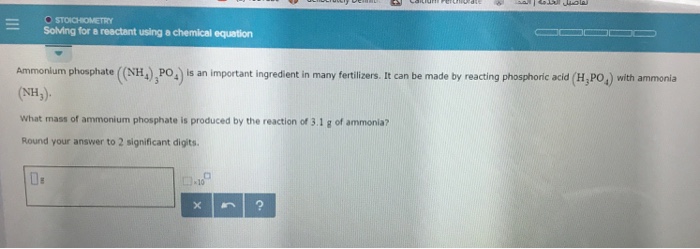
== O STOICHIOMETRY Solving for a reactant using a chemical equation Ammonium phosphate ((NH),PO) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia (NH). What mass of ammonium phosphate is produced by the reaction of 3.1 g of ammonia? Round your answer to 2 significant digits. DE X ?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


