Question: Someone knowledgable please help with part A, B, C and D. MISSED THIS? Read Suction 16.3 (Pagns 688 - 392) ThereactionbelowhasanequilibriumconstantKp=2.2106at298K.CalculateKpforthereactionbelow4COF2(g)=2CO2(g)+2CF4(g) 2COF2(g)=CO2(g)+CF4(g) Express your answer
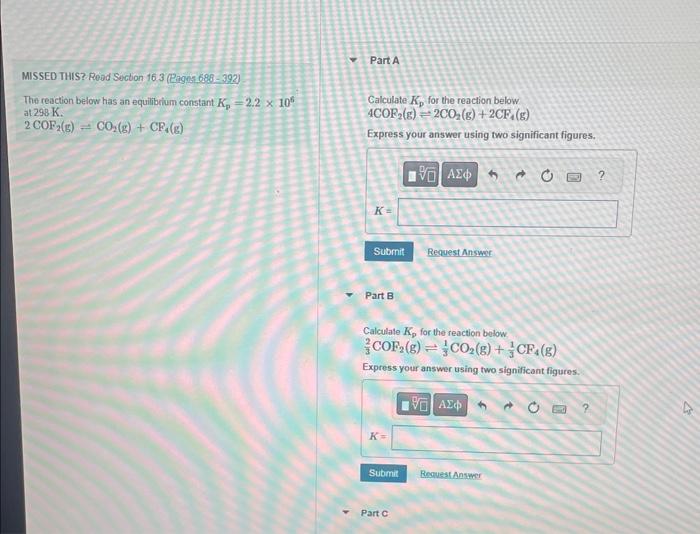
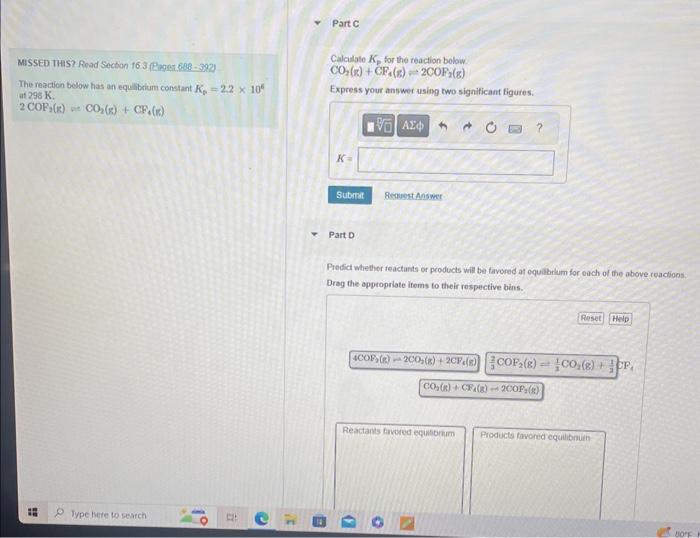
MISSED THIS? Read Suction 16.3 (Pagns 688 - 392) ThereactionbelowhasanequilibriumconstantKp=2.2106at298K.CalculateKpforthereactionbelow4COF2(g)=2CO2(g)+2CF4(g) 2COF2(g)=CO2(g)+CF4(g) Express your answer using two significant figures. Part B Calculate Kp for the reaction below 32COF2(g)31CO2(g)+31CF4(g) MISSED THIS? Road Section 16.3 fRaget 688 - 392 . Calculale Kp for the reaction bolow CO2(z)+CP4(s)2COP2(g) The reaction below has an equllibium constant K5=2.2106 at 290K. Express your answer using two significant figures. 2COP2(k)+CO2(g)+CP4(e) Part D Predict whether reactants or products will be tavored af equilbrlum for each of the above reactions. Drag the apptopriate items to their respective beins
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


