Question: Someone knowledgeable please help with the calculation part on my lab because im wasting questions without getting any help. This is a Colligative Property lab


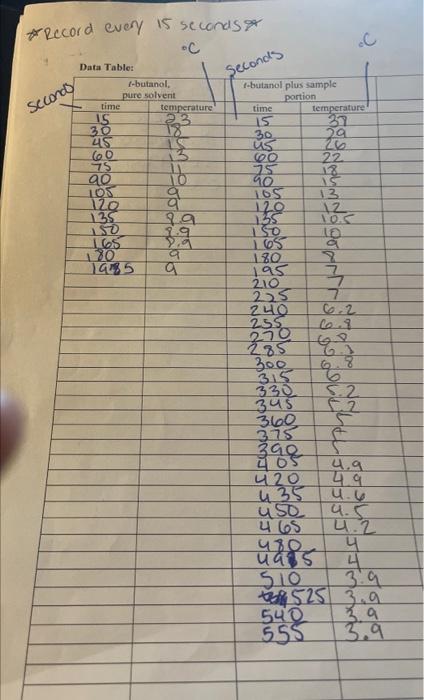
Data Handling, Calculations and Questions: Use graph paper and plot temperature vs. time for the pure t-butanol and for each solution analyzed; make 3 different graphs. Use the discussion in the background section above as a guide and determine the freezing point, Tf, of t-butanol and of each solution. Clearly mark on each graph all your data points and the best fit lines you used to determine freezing point. Determine the Tf of solution 1 by subtracting the Tf of solution 1 from the Tf of pure t-butanol. Tf of solution = Use the Tf for solution along with the mass of unknown in solution I, line 4 of the data table, the mass of solvent, t-butanol, line 3b of the data table, and the kf of t-butanol, 9.10Ckg solvent /mol solute, in Eq 6 to determine the molar mass, M, of your unknown compound. M, of your unknown compound = 1) Mass of test tube: 2) Mass of test tube and t-butanol: 3) a) Mass of t-butanol (line 2 - line 1 ): 9. 40000 b) Mass of t-butanol in kgs: .009ks 4) Mass of first sample of unknown: 0.64g * roarnid every 15 seconds Data Handling, Calculations and Questions: Use graph paper and plot temperature vs. time for the pure t-butanol and for each solution analyzed; make 3 different graphs. Use the discussion in the background section above as a guide and determine the freezing point, Tf, of t-butanol and of each solution. Clearly mark on each graph all your data points and the best fit lines you used to determine freezing point. Determine the Tf of solution 1 by subtracting the Tf of solution 1 from the Tf of pure t-butanol. Tf of solution = Use the Tf for solution along with the mass of unknown in solution I, line 4 of the data table, the mass of solvent, t-butanol, line 3b of the data table, and the kf of t-butanol, 9.10Ckg solvent /mol solute, in Eq 6 to determine the molar mass, M, of your unknown compound. M, of your unknown compound = 1) Mass of test tube: 2) Mass of test tube and t-butanol: 3) a) Mass of t-butanol (line 2 - line 1 ): 9. 40000 b) Mass of t-butanol in kgs: .009ks 4) Mass of first sample of unknown: 0.64g * roarnid every 15 seconds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


