Question: Specifically for question 2, I need a hand figuring out what voltage I am supposed to use for the anodes and cathodes. Please ignore question
Specifically for question 2, I need a hand figuring out what voltage I am supposed to use for the anodes and cathodes. Please ignore question 1, and if you can help me start with three that would be appreciated but 2 is the one I'm most stuck on.
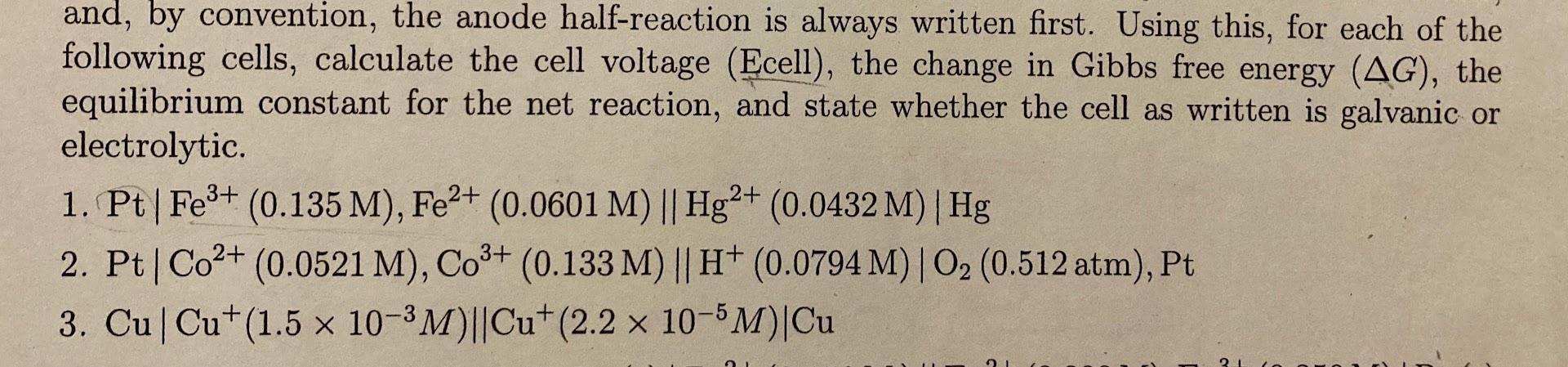
and, by convention, the anode half-reaction is always written first. Using this, for each of the following cells, calculate the cell voltage (Ecell), the change in Gibbs free energy (AG), the equilibrium constant for the net reaction, and state whether the cell as written is galvanic or electrolytic. 1. Pt|Fe3+ (0.135 M), Fe2+ (0.0601 M) || Hg2+ (0.0432 M) Hg | 2. Pt | Co2+ (0.0521 M), CO3+ (0.133 M) || H+ (0.0794 M)|O2 (0.512 atm), Pt 3. CuCu+(1.5 x 10-3M)||Cu+(2.2 x 10-5M)Cu 21
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


