Question: Standard Reduction Potentials The standard reduction potential, E, of any redox pair is defined for the half-cell reaction: Oxidizing agent + n electrons reducing
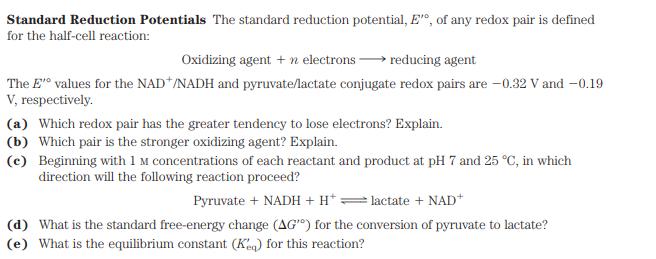
Standard Reduction Potentials The standard reduction potential, E", of any redox pair is defined for the half-cell reaction: Oxidizing agent + n electrons reducing agent The E' values for the NAD*/NADH and pyruvate/lactate conjugate redox pairs are -0.32 V and -0.19 V, respectively. (a) Which redox pair has the greater tendency to lose electrons? Explain. (b) Which pair is the stronger oxidizing agent? Explain. (c) Beginning with 1 M concentrations of each reactant and product at pH 7 and 25 C, in which direction will the following reaction proceed? Pyruvate + NADH + H+: lactate + NAD+ (d) What is the standard free-energy change (AG") for the conversion of pyruvate to lactate? (e) What is the equilibrium constant (Keq) for this reaction?
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


