Question: standerd deviation needed as well as part three in the second picture thank u Weight of potassium hydrogen ( KH) phthalate: 0.14150g Average concentration of
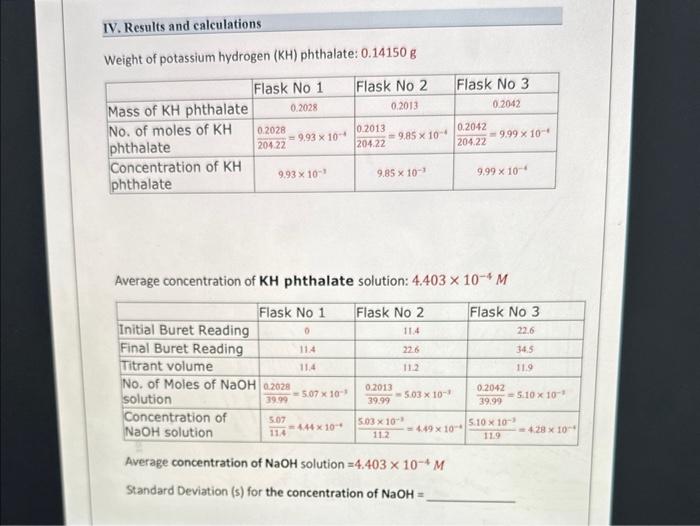
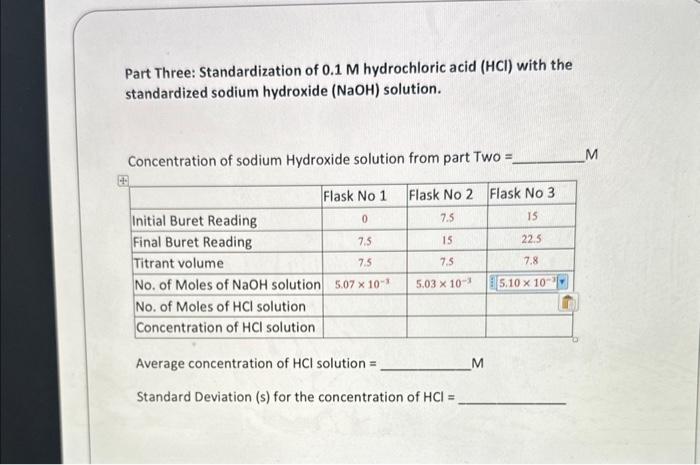
Weight of potassium hydrogen ( KH) phthalate: 0.14150g Average concentration of KH phthalate solution: 4.403104M Average concentration of NaOH solution =4.403104M Standard Deviation (s) for the concentration of NaOH= Part Three: Standardization of 0.1M hydrochloric acid ( HCl) with the standardized sodium hydroxide (NaOH) solution. Concentration of sodium Hydroxide solution from part Two = M Average concentration of HCl solution = M Standard Deviation (s) for the concentration of HCl=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


