Question: State the mole ratio between the reactants in the following balanced equation:4NaOH + 2F2 ---- ----> 4NaF + 2H2O + O2 * 2 points Your
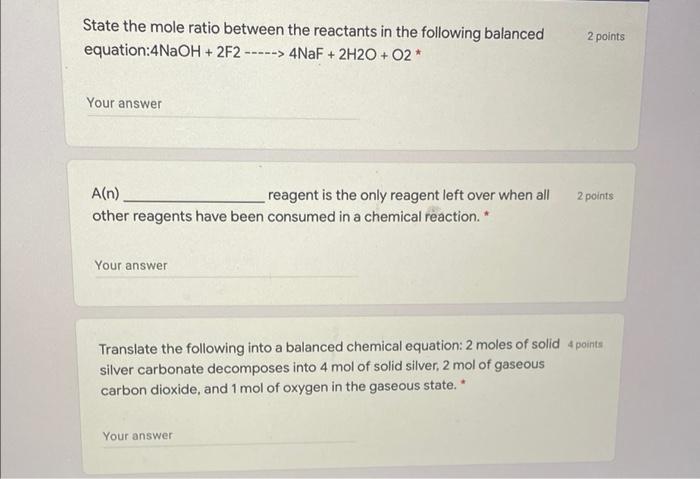
State the mole ratio between the reactants in the following balanced equation:4NaOH + 2F2 ---- ----> 4NaF + 2H2O + O2 * 2 points Your answer 2 points A(n) reagent is the only reagent left over when all other reagents have been consumed in a chemical reaction. Your answer Translate the following into a balanced chemical equation: 2 moles of solid 4 points silver carbonate decomposes into 4 mol of solid silver, 2 mol of gaseous carbon dioxide, and 1 mol of oxygen in the gaseous state. Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


