Question: step by step 15. Corey makes a solution by mixing 100.0mL of 0.200MBaCl2 and 150.0mL of 0.400M NaCl. Calculate the concentration of NaCl in the
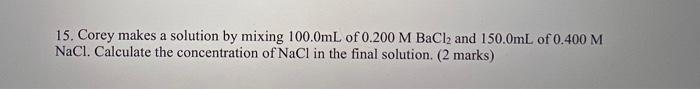
15. Corey makes a solution by mixing 100.0mL of 0.200MBaCl2 and 150.0mL of 0.400M NaCl. Calculate the concentration of NaCl in the final solution. ( 2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


