Question: step by step please 5. Silver ion forms the complex Ag(CN)2 with Kf=9.8 1021. What is the minimum amount of HCN that would need to
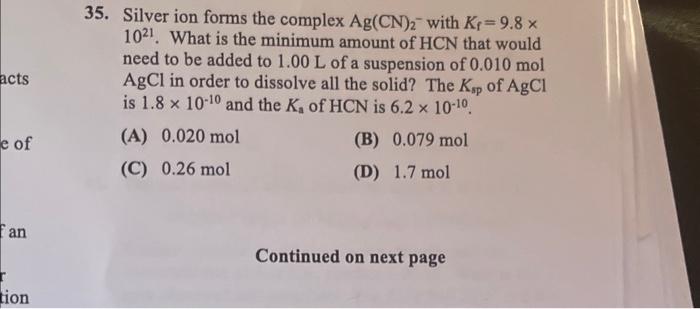
5. Silver ion forms the complex Ag(CN)2 with Kf=9.8 1021. What is the minimum amount of HCN that would need to be added to 1.00L of a suspension of 0.010mol AgCl in order to dissolve all the solid? The Ksp of AgCl is 1.81010 and the Ka of HCN is 6.21010. (A) 0.020mol (B) 0.079mol (C) 0.26mol (D) 1.7mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


