Question: step by step please Problems 1. Indicate whether the following statement is true or false and explain your reasoning. The value of Kc for the
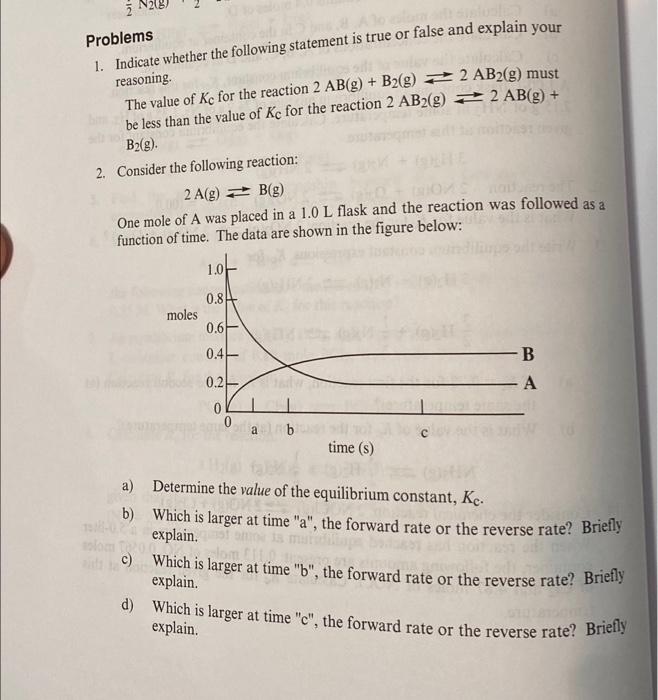
Problems 1. Indicate whether the following statement is true or false and explain your reasoning. The value of Kc for the reaction 2AB(g)+B2(g)2AB2(g) must be less than the value of Kc for the reaction 2AB2(g)2AB(g)+ B2 (g). 2. Consider the following reaction: 2A(g)B(g) One mole of A was placed in a 1.0L flask and the reaction was followed as a function of time. The data are shown in the figure below: a) Determine the value of the equilibrium constant, Kc. b) Which is larger at time "a", the forward rate or the reverse rate? Briefly explain. c) Which is larger at time "b", the forward rate or the reverse rate? Briefly explain. d) Which is larger at time " c ", the forward rate or the reverse rate? Briefly explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


