Question: step by step please The attached graph plots the (approximate) partial molar volumes of ethanol and water in units of cm3/ mole at 25C in

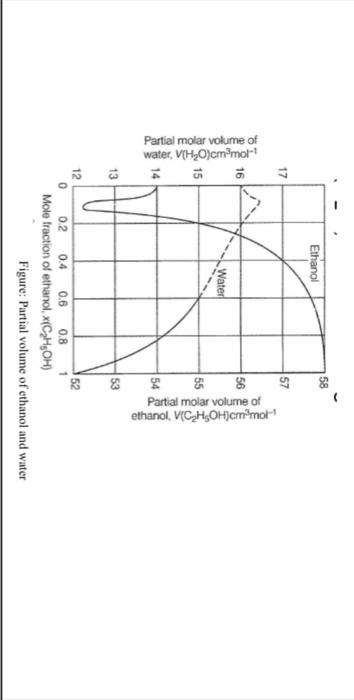
The attached graph plots the (approximate) partial molar volumes of ethanol and water in units of cm3/ mole at 25C in a mixture as a function of the mole fraction of ethanol. Use the information in this graph (note the different y axes for water and ethanol) and the densities at 25C to calculate the resulting total volume of the mixture when 150cm3 ethanol is mixed with 100cm3H2O. (Density of ethanol =0.789g/cm3 \& Density of water =0.998g/cm3 ). Also calculate the excess volume in this mixture. Figure: Partial volume of ethanol and water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


