Question: 3. The attached graph plots the (approximate) partial molar volumes of ethanol and in units of cm /mole at 25C in a mixture as a
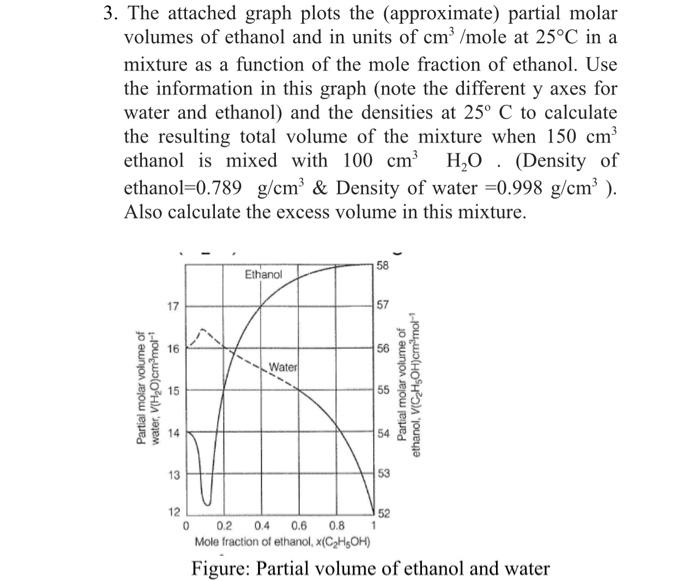
3. The attached graph plots the (approximate) partial molar volumes of ethanol and in units of cm /mole at 25C in a mixture as a function of the mole fraction of ethanol. Use the information in this graph (note the different y axes for water and ethanol) and the densities at 25 C to calculate the resulting total volume of the mixture when 150 cm ethanol is mixed with 100 cm H.O. (Density of ethanol=0.789 g/cm & Density of water =0.998 g/cm). Also calculate the excess volume in this mixture. 58 Ethanol 17 57 16 56 Water Partial molar volume of water, VIH em molt 55 Partial molar volume of ethanol (CH3OH)cm'mol 15 14 13 53 12 52 02 0.4 0.6 0.8 Mole fraction of ethanol (CHOH) Figure: Partial volume of ethanol and water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


