Question: step by step procedure, please A PEM type fuel cell presents the following electrochemical reactions: Anode 2H 4H+ + 4e Cathode 02 + 4H+ +
step by step procedure, please
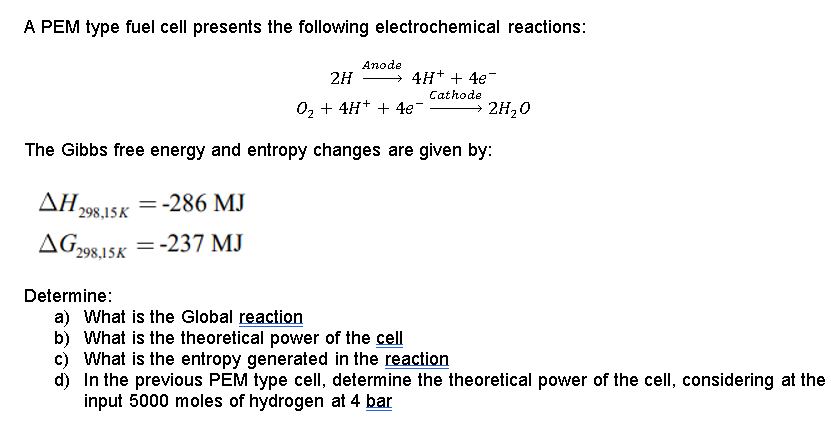
A PEM type fuel cell presents the following electrochemical reactions: Anode 2H 4H+ + 4e Cathode 02 + 4H+ + 4e- 2H20 The Gibbs free energy and entropy changes are given by: , 1298,15% = -286 MJ = AG298.15K = :-237 MJ Determine: a) What is the Global reaction b) What is the theoretical power of the cell c) What is the entropy generated in the reaction d) In the previous PEM type cell, determine the theoretical power of the cell, considering at the input 5000 moles of hydrogen at 4 bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


