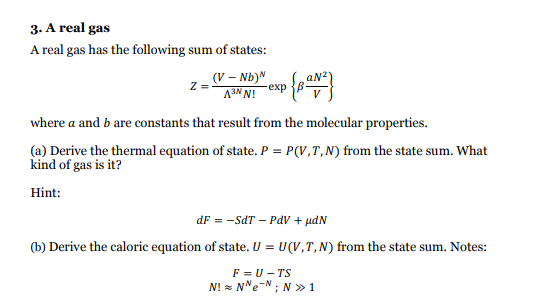
Question: step by step solution please -exp p{ream 3. A real gas A real gas has the following sum of states: (V - Nb) Z= ANN!
step by step solution please
-exp p{ream 3. A real gas A real gas has the following sum of states: (V - Nb) Z= ANN! where a and b are constants that result from the molecular properties. (a) Derive the thermal equation of state. P = P(V.T,N) from the state sum. What kind of gas is it? Hint: dF = -ST - POV + udN (b) Derive the caloric equation of state. U = U(V,T,N) from the state sum. Notes: F = U-TS N! Ne-N; N 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


