Question: steps to solve: 1. Generate Arrhenius Plot (log k vs 1/T) 2. Solve the Arrhenius Equation for Kapp at room temp (298 degrees kelvin). (LogA=Y
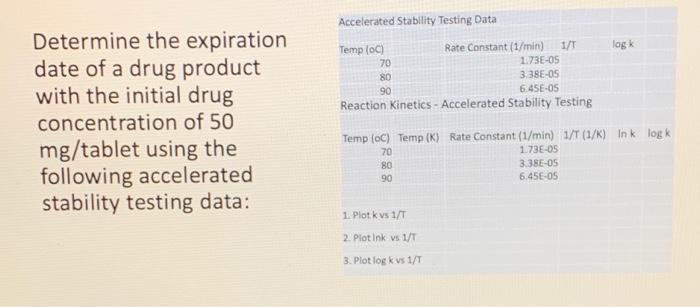
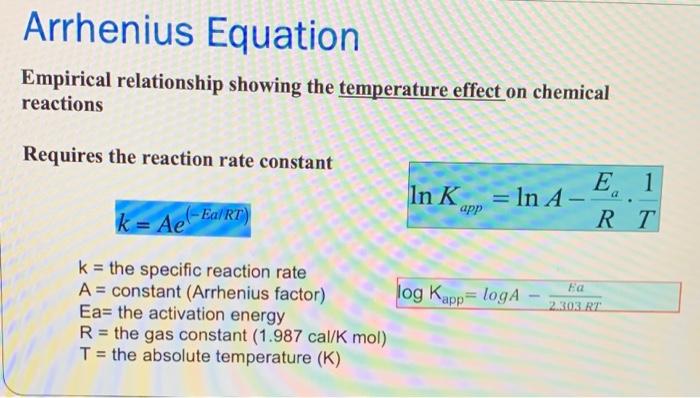

Accelerated Stability Testing Data log Temp (oC) Rate Constant (1/min) 1/1 70 1.73E-05 80 3.38E-OS 90 6.45E-05 Reaction Kinetics - Accelerated Stability Testing Determine the expiration date of a drug product with the initial drug concentration of 50 mg/tablet using the following accelerated stability testing data: Temp (oC) Temp (K) Rate Constant (1/min) 1/T (1/) Ink logk 70 173E-OS 80 3.38E-OS 90 6.45E OS 1. Plot k vs 1/T 2. Plot Ink vs 1/T 3. Plot log k vs 1/T Arrhenius Equation Empirical relationship showing the temperature effect on chemical reactions Requires the reaction rate constant In K = app E 1 = In A- RT k= Ael-Ea/RT) k = the specific reaction rate A = constant (Arrhenius factor) log Kapp= loga Ea= the activation energy R = the gas constant (1.987 cal/K mol) T = the absolute temperature (K) Ea 22303 RT Shelf-life = t30 -90 The time when 90% of the drug is remaining unchanged. A4 = 0.9A. tgo= 0.1A./k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


