Question: Stoichiometric problems can be tackled by remembering five basic steps. Step 1: Write a balanced chemical equation for the reaction. Step 2: List all data

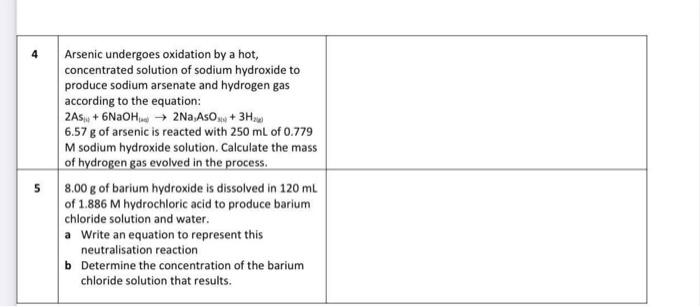
Stoichiometric problems can be tackled by remembering five basic steps. Step 1: Write a balanced chemical equation for the reaction. Step 2: List all data given, including relevant units. Remember to also write down the symbol of the unknown quantity. Step 3: Convert the data given to moles, using the relevant formulas: n=Mmn=cVn=NAN Step 4: Use the chemical equation to determine the mole ratio of the unknown quantity to the known quantity. This ratio enables calculation of the number of moles of the unknown quantity. Step 5: Finally, convert this number of mole back into the relevant units of the unknown. In all calculation questions, take care to show all steps in your working, including reacting ratios where relevant. Also, take care to add the correct unit to your answer (e.g. mol/L) and give your answer to the correct number of significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


