Question: Struggling with this concept. How do I determine a rate law? The reaction: 4A+B+C2A2B+2AC was studied at 25C. The following results were obtained: L1s1) a.
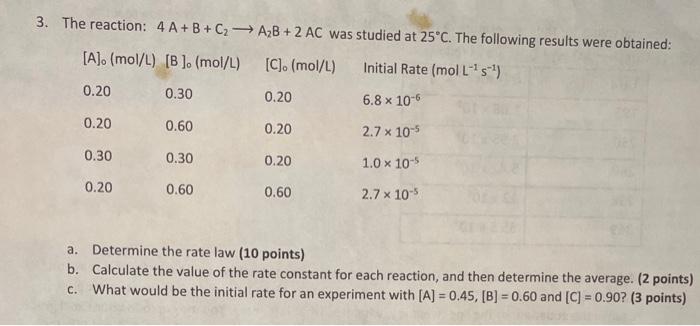
The reaction: 4A+B+C2A2B+2AC was studied at 25C. The following results were obtained: L1s1) a. Determine the rate law ( 10 points) b. Calculate the value of the rate constant for each reaction, and then determine the average. ( 2 points) c. What would be the initial rate for an experiment with [A]=0.45,[B]=0.60 and [C]=0.90 ? (3 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


