Question: Study the electrode potentials for the half cells below and use them to answer the questions that follow. The letters do not represent the
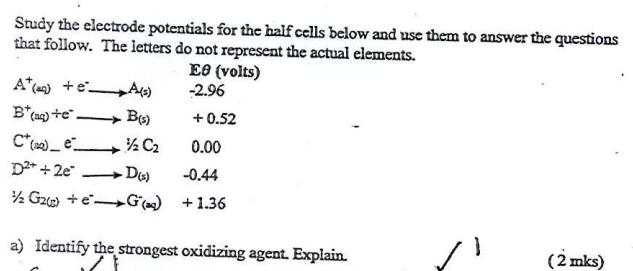



Study the electrode potentials for the half cells below and use them to answer the questions that follow. The letters do not represent the actual elements. A(+eA(=) B*(a)te. C*(a)_e__ D+ + 2e -D(s) 2 G2) +eG() B(s) C EO (volts) -2.96 +0.52 0.00 -0.44 +1.36 a) Identify the strongest oxidizing agent. Explain. (2 mks) b) Which of the two half cells would produce the highest potential difference when combined. / \(2 mks) c) Explain whether the reaction below can take place. D(s) +2A* (ag) D+ (aq) + 2A(s) d) Draw a well labelled diagram when combining A and B half cells. negative. (3 mks)
Step by Step Solution
3.50 Rating (143 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


