Question: Study the model below. Answer the following questions. Question 13 (1 point) The electron pair geometry of this molecule is linear trigonal planar tetrahedral The
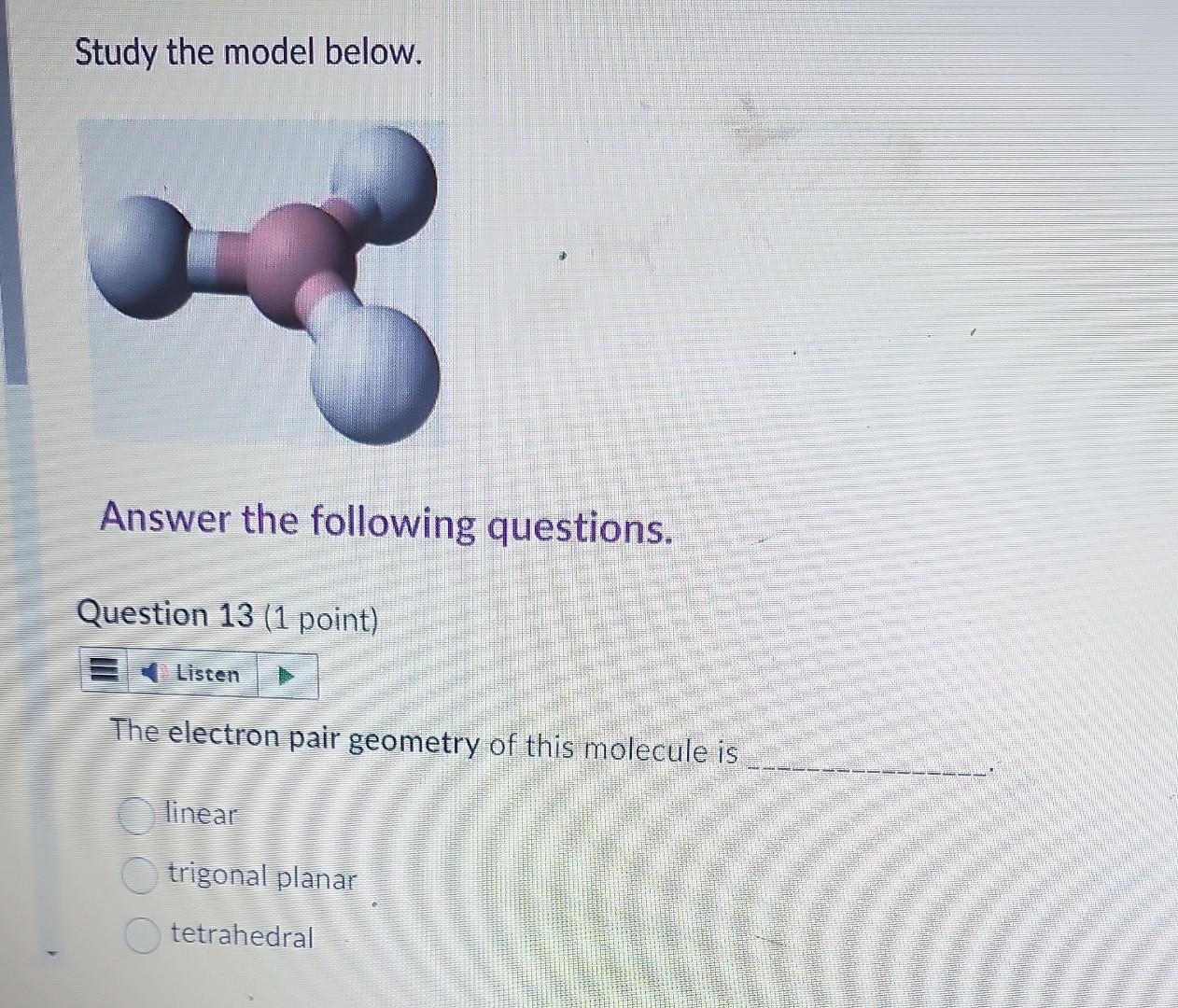
Study the model below. Answer the following questions. Question 13 (1 point) The electron pair geometry of this molecule is linear trigonal planar tetrahedral The molecular shape of this molecule is linear trigonal planar tetrahedral trigonal pyramidal V-shaped Question 15 (1 point) How many lone electron pair(s) is/are around the central atom? 0 1 2 The bond angle is 104.5107109.5120
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


