Question: Subject: Science, Physics, not General Physics. Topic: Density. Would you mind helping me answer these end of chapter questions on page 175 please? Please don't
Subject: Science, Physics, not General Physics. Topic: Density. Would you mind helping me answer these end of chapter questions on page 175 please? Please don't answer these numbers 7-14 on pink box and don't answer these densities of gases numbers 1-3, only end of chapter questions, thank you for your help.
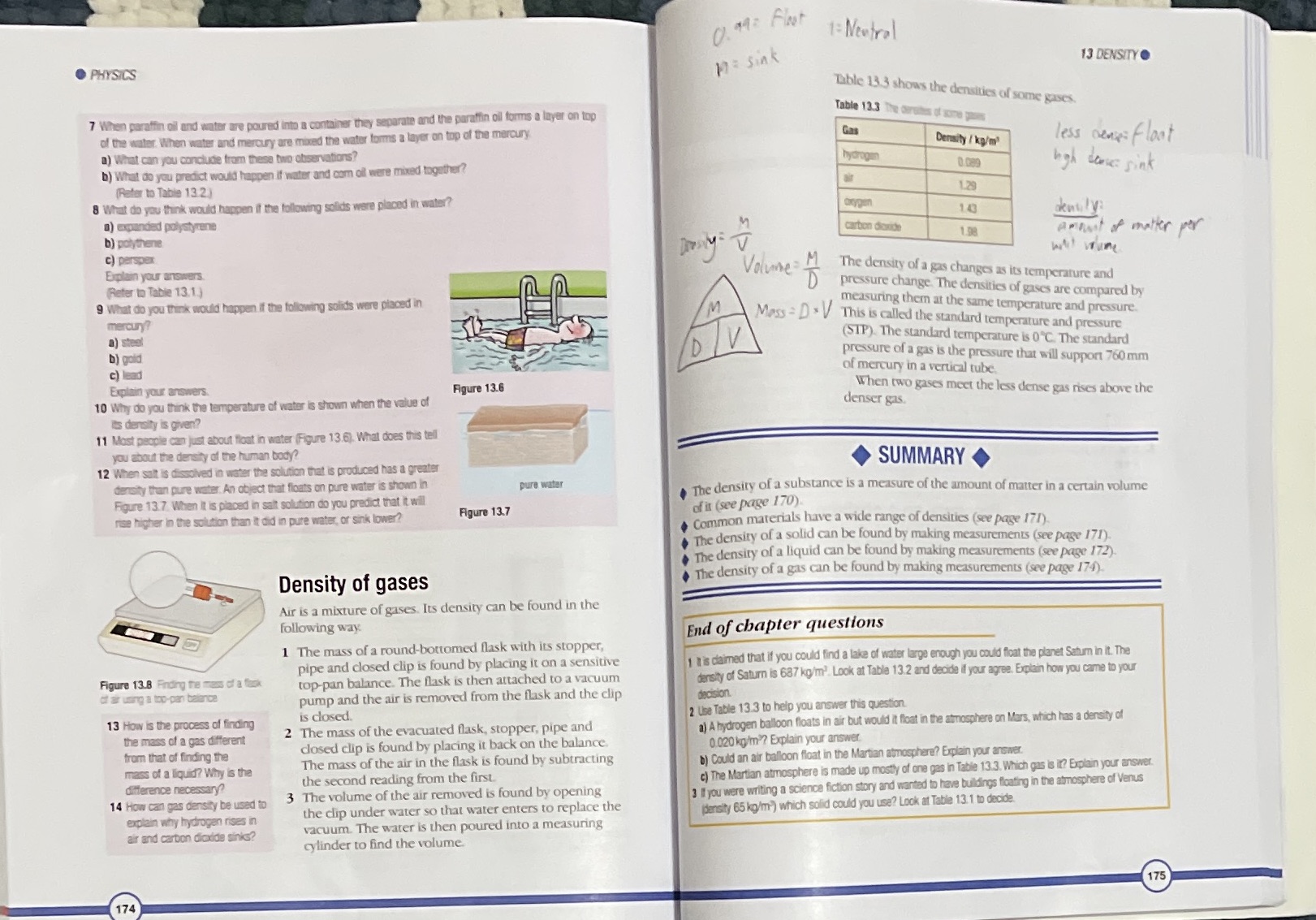
0 49 2 Floot 1: Neutral . PHYSICS 17 : sink 13 DENSITY . Table 13.3 shows the densities of some gases. 7 When paraffin oil and water are poured into a container they separate and the paraffin oil forms a layer on top Table 13.3 The berets of gone of the water. When water and mercury are mixed the water forms a layer on top of the mercury. Gar a) What can you conclude from these two observations? Density / ky/m' less sense: float b) What do you predict would happen if water and corn oil were mixed together? 0.089 high doses sink (Refer to Table 13.2) 8 What do you think would happen if the following solids were placed in water? 1.29 Oxygen 1) expanded polystyrene 183 dently b) polyhene carbon dioxide Amount of matter per c) perspex Explain your answers Volume: M (Refer to Table 13.1) D The density of a gas changes as its temperature and 9 What do you think would happen if the following solids were placed in pressure change. The densities of gases are compared by mercury? IM measuring them at the same temperature and pressure. V Mess = D* V This is called the standard temperature and pressure a) steel b) gold D (STP). The standard temperature is 0"C. The standard c) lead pressure of a gas is the pressure that will support 760 mm of mercury in a vertical tube. Explain your answers. Figure 13.6 10 Why do you think the temperature of water is shown when the value of denser gas. When two gases meet the less dense gas rises above the its density is given? 11 Most people can just about float in water (Figure 13.6). What does this tell you about the density of the human body? 12 When salt is dissolved in water the solution that is produced has a greater SUMMARY density than pure water. An object that floats on pure water is shown in pure water Figure 13.7 When It is placed in salt solution do you predict that it will The density of a substance is a measure of the amount of matter in a certain volume ise higher in the solution than it did in pure water, of sink lower? Figure 13.7 of it (see page 170) Common materials have a wide range of densities (see page 171). The density of a solid can be found by making measurements (see page 171). The density of a liquid can be found by making measurements (see page 172) Density of gases The density of a gas can be found by making measurements (see page 174). Air is a mixture of gases. Its density can be found in the following way. End of chapter questions 1 The mass of a round-bottomed flask with its stopper, pipe and closed clip is found by placing it on a sensitive is claimed that if you could find a lake of water large enough you could float the planet Saturn in it. The Figure 13.8 Finding the mess of a task top-pan balance. The flask is then attached to a vacuum density of Saturn is 687 ko/m'. Look at Table 13.2 and decide if your agree. Explain how you came to your of air using a top-pan balance pump and the air is removed from the flask and the clip decision. 13 How is the process of finding is closed. 2 Use Table 13.3 to help you answer this question. the mass of a gas different 2 The mass of the evacuated flask, stopper, pipe and a) A hydrogen balloon floats in air but would it float in the atmosphere on Mars, which has a density of from that of finding the closed clip is found by placing it back on the balance. 0.020 kg/m ? Explain your answer. mass of a liquid? Why is the The mass of the air in the flask is found by subtracting b) Could an air balloon float in the Martian atmosphere? Explain your answer. difference necessary? the second reading from the first. c) The Martian atmosphere is made up mostly of one gas in Table 13.3. Which gas is it? Explain your answer. 14 How can gas density be used to 3 The volume of the air removed is found by opening 3 I you were writing a science fiction story and wanted to have buildings floating in the atmosphere of Venus explain why hydrogen rises in the clip under water so that water enters to replace the (density 65 kg/m ) which solid could you use? Look at Table 13.1 to decide. air and carbon dioxide sinks? vacuum. The water is then poured into a measuring cylinder to find the volume. 175
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


