Question: Subject: Science, Topic: Density. Would you mind helping me answer questions 7-14 from the pink box please? And also answer the End of chapter questions,
Subject: Science, Topic: Density. Would you mind helping me answer questions 7-14 from the pink box please? And also answer the "End of chapter questions", except "Density of Gases". This is other than General Physics. If you can't find the answers, you may research. Thank you for your help.
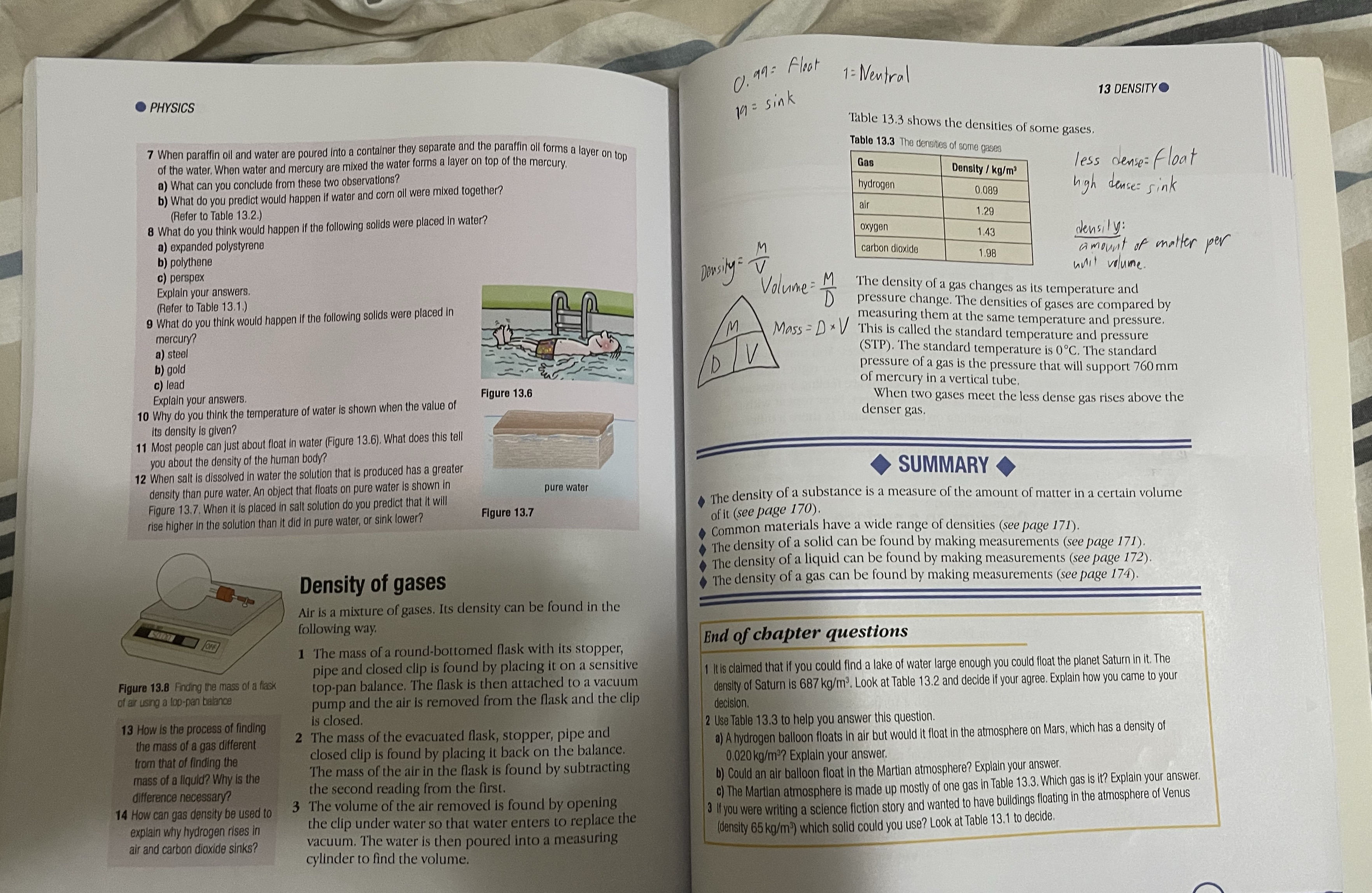
0. 99 : Float 1: Neutral PHYSICS 13 DENSITY 19 = sink Table 13.3 shows the densities of some gases, When paraffin oil and water are poured into a container they separate and the paraffin oil forms a layer on top Table 13.3 The densities of some gases of the water. When water and mercury are mixed the water forms a layer on top of the mercury. Gas a) What can you conclude from these two observations? Density / kg/m' less dense= Float b) What do you predict would happen if water and corn oil were mixed together? hydrogen 0.089 high dense : sink (Refer to Table 13.2.) air 8 What do you think would happen if the following solids were placed in water? 1.29 oxygen a) expanded polystyrene 1.43 densily : b) polythene carbon dioxide Density = M 1.98 amount of matter per c) perspex unit volume. Explain your answers Volume = M (Refer to Table 13.1.) The density of a gas changes as its temperature and 9 What do you think would happen If the following solids were placed In pressure change. The densities of gases are compared by mercury M measuring them at the same temperature and pressure. a) steel Mass= D * V This is called the standard temperature and pressure b) gold D (STP). The standard temperature is 0 C. The standard c) lead pressure of a gas is the pressure that will support 760 mm Explain your answers. Figure 13.6 of mercury in a vertical tube. 10 Why do you think the temperature of water is shown when the value of When two gases meet the less dense gas rises above the denser gas. Its density is given? 11 Most people can just about float in water (Figure 13.6). What does this tell you about the density of the human body? 12 When salt is dissolved in water the solution that is produced has a greater SUMMARY density than pure water. An object that floats on pure water Is shown in pure water Figure 13.7. When it is placed in salt solution do you predict that it will Figure 13.7 The density of a substance is a measure of the amount of matter in a certain volume rise higher In the solution than it did In pure water, or sink lower? of it (see page 170) Common materials have a wide range of densities (see page 171). The density of a solid can be found by making measurements (see page 171). The density of a liquid can be found by making measurements (see page 172) Density of gases The density of a gas can be found by making measurements (see page 174). Air is a mixture of gases. Its density can be found in the following way. 1 The mass of a round-bottomed flask with its stopper, End of chapter questions pipe and closed clip is found by placing it on a sensitive 1 It is claimed that if you could find a lake of water large enough you could float the planet Saturn in it. The Figure 13.8 Finding the mass of a flask top-pan balance. The flask is then attached to a vacuum of air using a top-pan balance density of Saturn is 687 kg/m'. Look at Table 13.2 and decide if your agree. Explain how you came to your pump and the air is removed from the flask and the clip decision. 13 How is the process of finding is closed. 2 Use Table 13.3 to help you answer this question. the mass of a gas different 2 The mass of the evacuated flask, stopper, pipe and a) A hydrogen balloon floats in air but would it float in the atmosphere on Mars, which has a density of from that of finding the closed clip is found by placing it back on the balance. The mass of the air in the flask is found by subtracting 0,020 kg/m'? Explain your answer. mass of a liquid? Why is the difference necessary? the second reading from the first. b) Could an air balloon float in the Martian atmosphere? Explain your answer. ") The Martian atmosphere is made up mostly of one gas in Table 13.3, Which gas is it? Explain your answer. 14 How can gas density be used to 3 The volume of the air removed is found by opening If you were writing a science fiction story and wanted to have buildings floating in the atmosphere of Venus explain why hydrogen rises in the clip under water so that water enters to replace the (density 65 kg/m') which solid could you use? Look at Table 13.1 to decide. air and carbon dioxide sinks? vacuum. The water is then poured into a measuring cylinder to find the volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


