Question: substitute E5 into E2 to get: E6: q=V(DwDi)DiDwHf This equation means that you can calculate the heat change of a reaction easily by measuring the

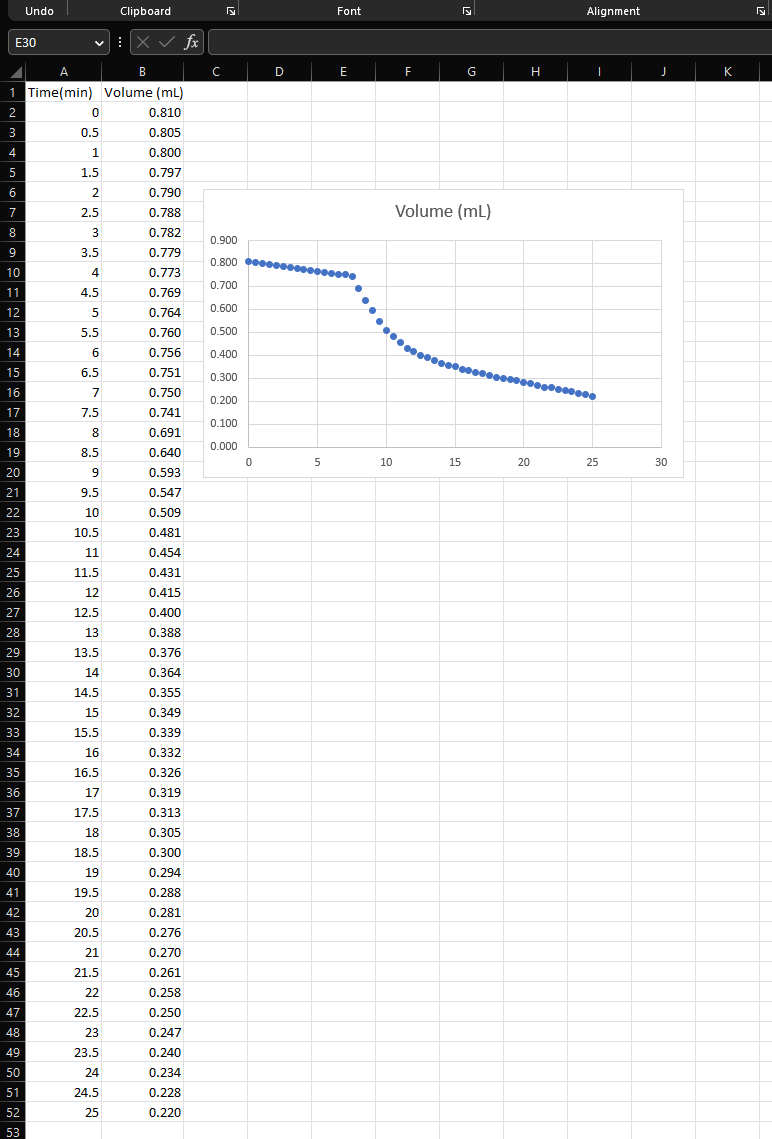

substitute E5 into E2 to get: E6: q=V(DwDi)DiDwHf This equation means that you can calculate the heat change of a reaction easily by measuring the change in volume of the icewater mixture in your calorimeter. CONCEPTUALIZE! Since a sample of ice occupies (more / less) space than the same weight of liquid water, the final volume measured will be (more / less) than the initial volume and V will be (positive / negative). This will also result in a (positive / negative) value for q, which is expected since heat is (released / absorbed) by an (exothermic / endothermic) reaction. The heat per mole (H) of Mg reacted is: E7: H=yqJ/mol where y is the number of moles of Mg reacted in the experiment Higure 1: ice caiorimeter setup The accuracy of any calorimeter depends on Crumple some paper towels and pack them being able to minimize heat exchange between in the bottom of a 1000mL beaker. all other sources except for the reaction in Thoroughly dry the inside of the test tube question. To do this, you will insulate your ice that is a part of the stopper assembly using a calorimeter as much as possible from the heat Kim Wipe wrapped around a pen. of the room with paper towels, water, and ice. Add 5mL of 2.0MHCl and stopper the test After your setup has been assembled (without tube tightly. any air bubbles inside the beaker), and acid has Fill the 200mL beaker 3/4 full of crushed been added to the test tube, the entire apparatus ice, then add cold water until it overflows. must come to equilibrium and sit for 10 Gently stir the 200mL beaker with a glass minutes before you start to take readings. rod to allow as many air bubbles to escape as possible. DATA: Don't forget to attach your data ANALYSIS: 1. On separate sheets, plot volume vs time for each trial. Read the "Detail of the Experiment" section of Experiment 3 in the lab manual to get an idea of what you are graphing. 2. Use Equations (6) and (7) to calculate H for Mg reacting with HCl. Show which points on the graph were used to determine V. Round H to the maximum number of significant figures allowed by your measurements. substitute E5 into E2 to get: E6: q=V(DwDi)DiDwHf This equation means that you can calculate the heat change of a reaction easily by measuring the change in volume of the icewater mixture in your calorimeter. CONCEPTUALIZE! Since a sample of ice occupies (more / less) space than the same weight of liquid water, the final volume measured will be (more / less) than the initial volume and V will be (positive / negative). This will also result in a (positive / negative) value for q, which is expected since heat is (released / absorbed) by an (exothermic / endothermic) reaction. The heat per mole (H) of Mg reacted is: E7: H=yqJ/mol where y is the number of moles of Mg reacted in the experiment Higure 1: ice caiorimeter setup The accuracy of any calorimeter depends on Crumple some paper towels and pack them being able to minimize heat exchange between in the bottom of a 1000mL beaker. all other sources except for the reaction in Thoroughly dry the inside of the test tube question. To do this, you will insulate your ice that is a part of the stopper assembly using a calorimeter as much as possible from the heat Kim Wipe wrapped around a pen. of the room with paper towels, water, and ice. Add 5mL of 2.0MHCl and stopper the test After your setup has been assembled (without tube tightly. any air bubbles inside the beaker), and acid has Fill the 200mL beaker 3/4 full of crushed been added to the test tube, the entire apparatus ice, then add cold water until it overflows. must come to equilibrium and sit for 10 Gently stir the 200mL beaker with a glass minutes before you start to take readings. rod to allow as many air bubbles to escape as possible. DATA: Don't forget to attach your data ANALYSIS: 1. On separate sheets, plot volume vs time for each trial. Read the "Detail of the Experiment" section of Experiment 3 in the lab manual to get an idea of what you are graphing. 2. Use Equations (6) and (7) to calculate H for Mg reacting with HCl. Show which points on the graph were used to determine V. Round H to the maximum number of significant figures allowed by your measurements
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


