Question: substitutional solid solutions related, (i) Plot how the enthalpy, entropy and the total free energy vary for these two systems as you change the composition
substitutional solid solutions related, (i) Plot how the enthalpy, entropy and the total free energy vary for these two systems as you change the composition by substitutional defects. In which of these two cases, substitution is endothermic or exothermic process?
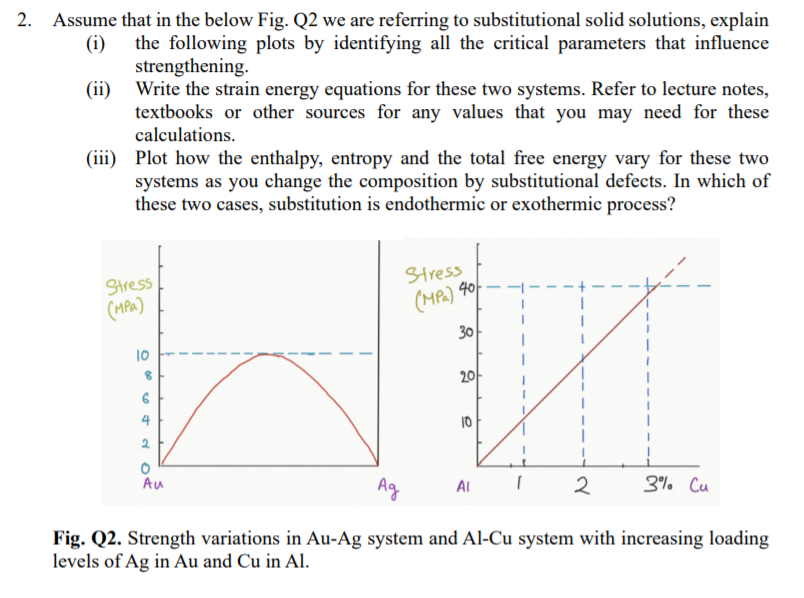
2. Assume that in the below Fig. Q2 we are referring to substitutional solid solutions, explain (i) the following plots by identifying all the critical parameters that influence strengthening (ii) Write the strain energy equations for these two systems. Refer to lecture notes, textbooks or other sources for any values that you may need for these calculations. (iii) Plot how the enthalpy, entropy and the total free energy vary for these two systems as you change the composition by substitutional defects. In which of these two cases, substitution is endothermic or exothermic process? stress Stress (MPa) (MPa) 40. I 30 10 8 20 6 4 10 1 2 0 Au Ag AI 2 3% Cu Fig. Q2. Strength variations in Au-Ag system and Al-Cu system with increasing loading levels of Ag in Au and Cu in Al
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


