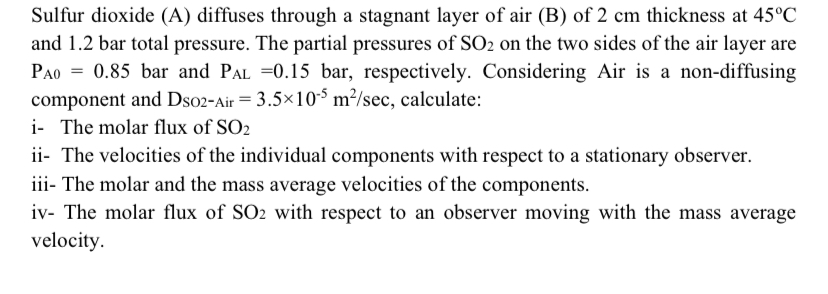
Question: Sulfur dioxide ( A ) diffuses through a stagnant layer of air ( B ) of 2 c m thickness at 4 5 C and
Sulfur dioxide A diffuses through a stagnant layer of air B of thickness at and bar total pressure. The partial pressures of on the two sides of the air layer are bar and bar, respectively. Considering Air is a nondiffusing component and DSOAir calculate:
i The molar flux of
ii The velocities of the individual components with respect to a stationary observer.
iii The molar and the mass average velocities of the components.
iv The molar flux of with respect to an observer moving with the mass average velocity.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


