Question: Sulfur dioxide and nitrogen dioxide react at 298K via the reaction SO2(g)+NO2(g)SO3(g)+NO(g) A system at equilibrium contains 0.364molSO2,0.364molNO2,0.711molSO3, and 0.643molNO2 is added to the container
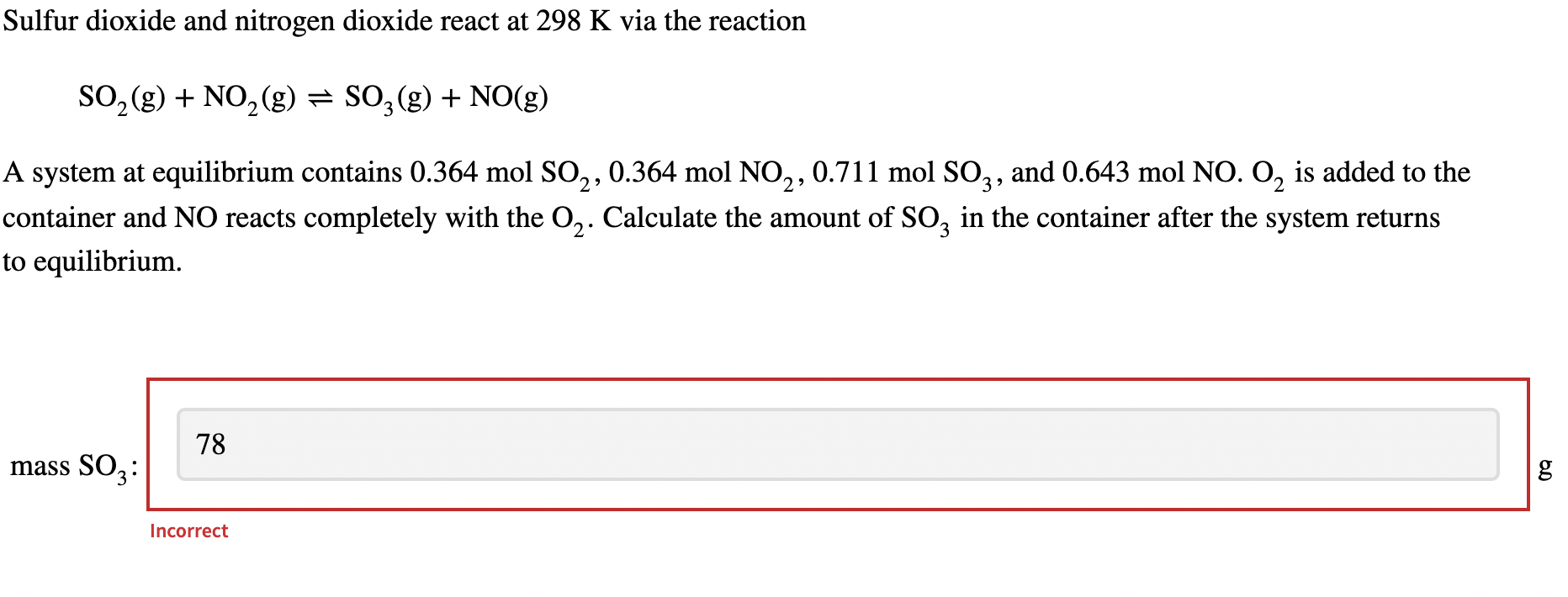
Sulfur dioxide and nitrogen dioxide react at 298K via the reaction SO2(g)+NO2(g)SO3(g)+NO(g) A system at equilibrium contains 0.364molSO2,0.364molNO2,0.711molSO3, and 0.643molNO2 is added to the container and NO reacts completely with the O2. Calculate the amount of SO3 in the container after the system returns to equilibrium. mass SO
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


