Question: summarize what to do on the 'UV-Absorption Spectroscopy Method. Write step by step how to do the lab and explain the result Determination of Critical


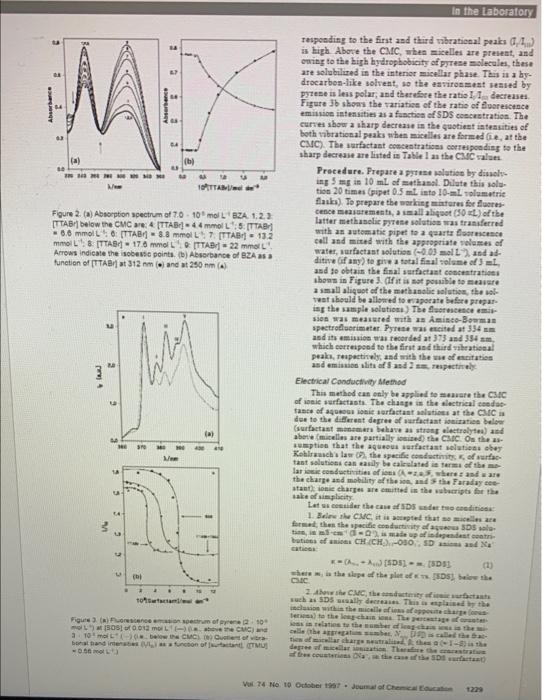
summarize what to do on the 'UV-Absorption Spectroscopy Method. Write step by step how to do the lab and explain the result Determination of Critical Micelle Concentration of Some Surfactants by Three Techniques Ana Dominguer. Aurora fernainder, Noemi Genriler, Emilia lglenias, "and Luin Montenegro Departamento de Quimica Fundamental e lndustiat. Facullad de Ciencias. Univeruidad de La Conuina 15071-La Coruaba. Spain Sarfactants, wematian called a fare acthe arcetr or dererout, ate amest the net weratile cheaicals atalable. Thry hast apglicabies in maty areas, inchating chameiatry fichemical his otck er equilitia), biolor (as mamhesse mametici), asd pharmace (h) Sarfictants art anghophilie materiale contaiting beth apolar long-chain hydracarbon Tail and polar, uiealby ienic, brad' procpe. In polar aolvasts. for example =ater, thin deal character of the amphiphale leade to atlf atsociutiae or mien lituties. the ouftastant meleceles afrange thamanhw igte arrasiead phobic part of the a gretate farmes the core of the micellic, while the petar haad sreeps are located at the mic+lle-ertor ietarface is coetact mith and hydrated by a asmber at water meloceles. Dopentiag es the clesmital utractare of the sarfactaat, its mictlit can be catianit, asiosic, a mophelitie (ruittariaaid), of aoticnic. This asiqae peogerty Eierekaterogunisus motin, that is, they are batengeasea oe a eicresespic ucale, nom theafk thin are of on hesefe. tyiliteriam Deupitu their greaing imgertasce, microbeturgaswees trate the ue ef raribas technigens to masare the CMC (2) 5). The experimests deucribed is thit sttad i mare deviresed. te familiaries ofedeati of phyaical ehamistry anth mictllar iolstiens and the bave atrattaral paramatern of aceilis. raterias waei to obierie the chases in zhpical and chasi fotmed. The apes pt meats the CMCC detarnisaties of ates. wirfactants of throe metbods. The atrastage and diakvantager of ack eas arn iadasatet. luat by maseriat a thasge at 0 the tretis spetria of Prea esoseath, and (ad) elhe aikctrical caefuctinity of at iosic iurfactast seluties as ibe ceacestration of the 13. Leberatery experiments, depentieg oe the hime atd eqcipment arailable. Each rntep of otsiant desit determine the Clte ef a sarfoctast, fir azample apdrea doderyl valite, by uaieg the tlirae laciagees ander the ane eb: Terinestal conditions. Tha ties roguired fur delerminsties of a CSIC talee by any of the lethaigoes fouerited hate is bocrs. The vesuramy of utadesti' reiklits and respecue to the Moterials experiautb imete teterallo goel. Con our suractants wedisa dedecyt- aulfure Gifma srodnctu and wort wand an acect of. (Tf porked Results It is noticed that a remarkable enkancemen UV-Absorption Spectroscopy Method absorption occuri just abore the CMIC. The conc This method is based on the tautomerism of correoponding to the break points obserted for ta benzoylacetone or 1-phenyl-1,3-batadione. Ketones and \& are tabulated in Table 1 . diketonet, such as BZA, can exist in two tautomeric forms: the ketonic and the enolic formu. In solution, BZA enolites Procedure. To obtain the data shown in Fig: practically exclusively to the cis-enolic form, which exists and Table 1, prepare a concentrated solutio in a cyclic configuration stabilized by intramolecular hydromgmL ) of BZA in dioxane (or another water. jta bending: cible nenpolar solvent). From this atock sole (+0.03 mol L1) propare an aqueous BZA aolu by pipetting 0.40mL into 25 -mL volumetric f. and diluting to the mark with water. To draw acan of the ipectrum, 0.40mL of the aqueous solution is tranaferred with a pipet to a 1.0 quarte cell together with the appropriate ame This stabilization of the enol will be more prenounced when of uurfactant, the additive (if any) and the to istermolecular hydrogen bonding with the solveat does not volame necesary to five a 3-mL total solume compete. Hence kete-enol aquilibria of BZA are extremely the cell ([BZA]7.103 mol . Th. The reforence 1olvent sensitive and the proportion of the enolic form in ibould coutain the same concestrations of sur? mueh freater in nonpolat solvents, sel as cyclole exane, tast and additive as the sample. The spectrum than in polar or bydrogen-bond denor solvents, , such as waBZA is the presence of varying surfactant cone ter of alcohols. trations can be measared with any UThis ipect BZA exists in water as a mixtare of both tavomers (the photemater. percestage of the enol tautemer has been measured as Fluorescence Spectroscopy Method 37.5\% (7). When a surfactant is added to a water solution This metbod is based on the solvent dopezdese of BZA, the amount of anel increases abruptly when the sur- brational basd intensities in pyrese moncemer fluore factant coneentration rikes abote the CMC because the Pyrene is one of the few condensed aromatic lydroe: molic Gorm is taken up by the micellas, which proside a less that show sigsificast fine structure (tibrational ban polar solvent than the aqueous phase. Figare 2a shows the effect of increasing surfactant con- sities of the variens vibrational bands of pyrese she cestration on the UVabsorption spectrum of aqueos BZA strong dependence on solrent environment ( O ). Boths solutions of 7,0+101molL1. Below the CMC no changes dipole moment and dielectric constasts were found are observed in the presence of surfictant nenomers, abere important in thin effect. the CMCC the absorption band centered at =312nm (due Figure 3a presents representative monomer Ile to the epolic form [7] is augmented; asd in parallel, the cence ipectra for pyrene (in vary dilate solations, [py. absorption band centered at =250mm (mainly due to the =210+molL-, so that complieations due to excime keto form) diminishes. The changes is the absorbasce sot aniee) in aqeecus sodiam dodecyl sulfate (SDS) solu meavarements at these two wavelengths with raryier con- above and below the CMC. Is the absence of micelles (o centration of surfactaat in aqueous solutions are ihown in the CMC) pyrene senses the polar *stironmeat of whe Figure 2b. molecules, the ratio of fluerevestace emiavion intensities is high Abore the CMC, nhen micellei are preient, and ouing to the high hydropbobicity of pyrest nsiecales, these art selabilited in the intenor micellar phase. This is a bydrocarben-like solvent, so the eavironment seased by pyrese is leas polar, and thereftre the ratis 1. I decreases. Figare Ib sheas the tariatice of the ratio of elvorestance eminion intensities as a fancties ef SDS coecrstration. The curnes abow a sharp decrease in the quotiont iatessities of both vobrational praks when micelles are formed (ie, at the C.CC). The iarfactast ecnseatratives eorrespoeding to the aharp decrease are linted is Table 1 an the CMCC ralaes. Precedere. Prepare a protat moletion by diualhing 5mg in 10ml of =4thanal. Dilate this sola. tion 20 times (ripet 0.5mt iste 10m tolumentrit flaski). To prepare the warkitg minteres for Ilaores= 6.6 mmol L-: :[ [TABI] = 8.8 mmol Lt, 7. [TTAEI] =13.2 with an autematic pipet to a cearte Ilstraceset funetion of [TTAar] at 312nm() and at 290nm (*) ditine (of any) te give a tatal fieal solame of I=L. asd se obtain the final aurfactazt coscentratioes ibese in Figure 3 . (ff it an not pouible to meavere a small aliquet of the mothanolic aolutics, the askweat ibould be allowntd to napocate befere proparis the umple solvtices.) The Alsernesence emijsies was measted with an Amiste-Booman apectrollaerimater. Pyrete wai ereited at 334mm asd ith enciaies aa teoorded at 373 and 354am, whick eerreipesed to the firnt and third vileatiseal peake, reupectivels, and with the uwe of encitation asd emiano ulith of 5 asd 2 an reapectively Electrical Conductivib Mlethod This method ean ealy be applind to sasare the C3sc of ionic anfatrasts. The claste in the sinctrical caedattaste of aquow ioaic iarfastant anlations at the Clic is den to the diferent depree of axrfactast iveiraties beleer (ourfactast mainemats belave as strest plectrolytea) asd abere (micellin are partially iceized) the CMtC. On the asaemption that the aqueoun asfactast selatieas oher tast solutices can aasily be calrelated it tarma of tbr methe ekarge and mobility of the ane, and in ale Faraday seeatantl ionie charges are emitted in the vulacripts for the ake of uinplicity Lat wi eesuliler the caus af IDS cuder twe cesdities: 1. Srirw the CMC. it is acepptet that as aicelles ace botiena of anices CH (CH) ,010;,50 asiane and N ' catice: x=(asxw)[SDS]==[DDS1 Whene m, in the slepe of the slat of Te. ISDSD teires ate uek ai SDs weallt decreaus. Thin a aplained by the Vus 74 N4 10 Ortober tiet? deamat of Chencal Eouraten the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


