Question: Suppose a 250. ml flask is filled with 0.80 mol of H, and 2.0 mol of HI. The following reaction becomes possible: H2(g) +12(8) 2HI(g)
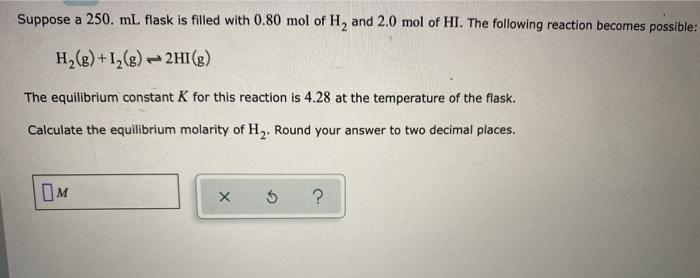
Suppose a 250. ml flask is filled with 0.80 mol of H, and 2.0 mol of HI. The following reaction becomes possible: H2(g) +12(8) 2HI(g) The equilibrium constant K for this reaction is 4.28 at the temperature of the flask. Calculate the equilibrium molarity of H. Round your answer to two decimal places. X 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


