Question: Suppose a 250. mL flask is filled with 1.5 mol of H20, 1.8 mol of CO2 and 0.60 mol of H2. The following reaction becomes
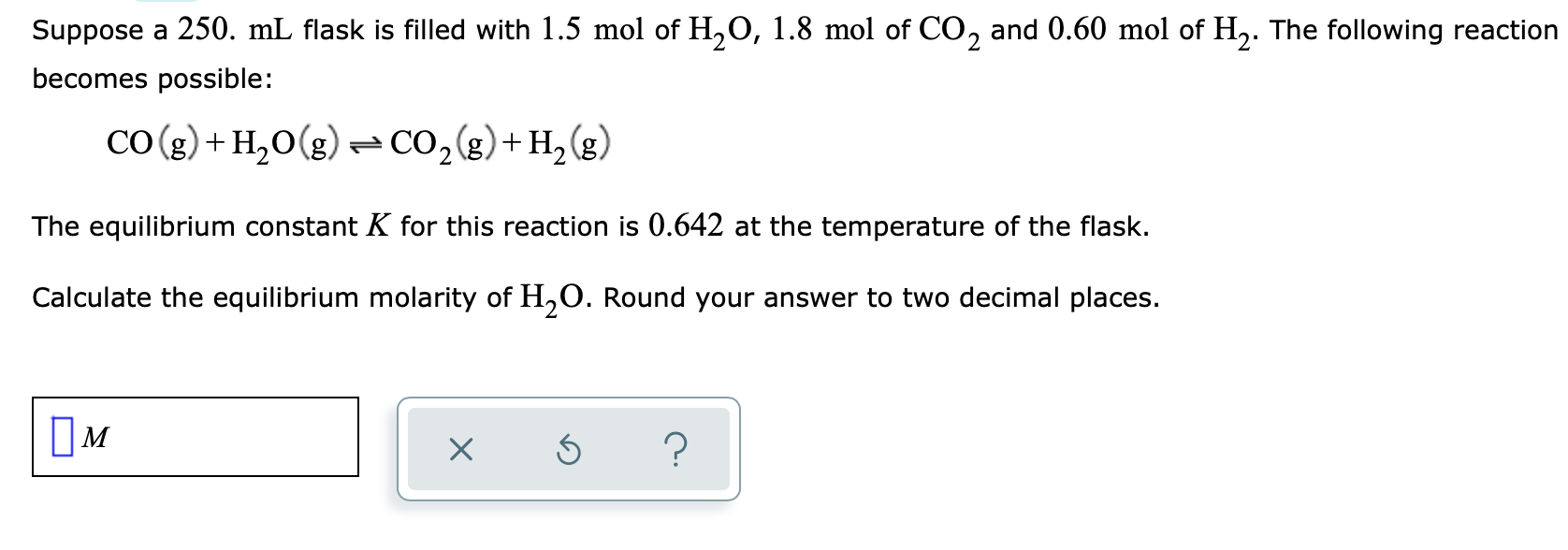
Suppose a 250. mL flask is filled with 1.5 mol of H20, 1.8 mol of CO2 and 0.60 mol of H2. The following reaction becomes possible: CO(g) + H2O(g) = CO2(g) + H2(g) The equilibrium constant K for this reaction is 0.642 at the temperature of the flask. Calculate the equilibrium molarity of H20. Round your answer to two decimal places. IM X 5
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


