Question: Suppose a student prepares reaction mixture 2 (see Table below). When the contents of the reaction flasks 1 and 2 are mixed together it takes
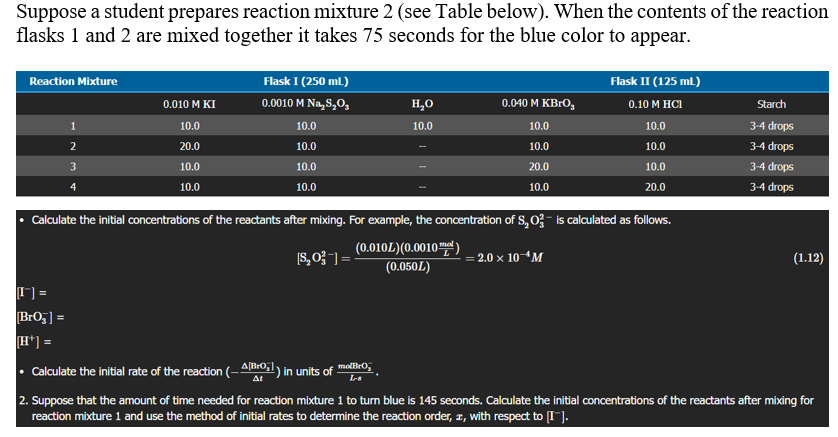
Suppose a student prepares reaction mixture 2 (see Table below). When the contents of the reaction flasks 1 and 2 are mixed together it takes 75 seconds for the blue color to appear. Reaction Mixture Flask I (250 mL) 0.0010 M Na,3,0, Flask II (125 mL) | 0.10 M HCl 0.010 M KI 0.040 M KBrog Starch HO 10.0 1 10.0 10.0 10.0 10.0 3-4 drops 3-4 drops 2 20.0 10.0 10.0 10.0 3 10.0 10.0 20.0 10.0 3-4 drops 4 10.0 10.0 10.0 20.0 3-4 drops Calculate the initial concentrations of the reactants after mixing. For example, the concentration of S, 03- is calculated as follows. (0.010L)(0.0010 me!) [S, 03-) = = 2.0 x 10 *M (1.12) (0.050L) T"]= Broj] = H+] = Calculate the initial rate of the reaction (-4/B20,1) in units of moBrO; 2. Suppose that the amount of time needed for reaction mixture 1 to turn blue is 145 seconds. Calculate the initial concentrations of the reactants after mixing for reaction mixture 1 and use the method of initial rates to determine the reaction order, I, with respect to [I). . At
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


