Question: Suppose that the root - mean - square velocity v r m s of water molecules ( molecular mass is equal to 1 8 .
Suppose that the rootmeansquare velocity of water molecules molecular mass is equal to in a flame is found to be
The Boltzmann constant is Avogadro's number is
What is the temperature of the water molecules?
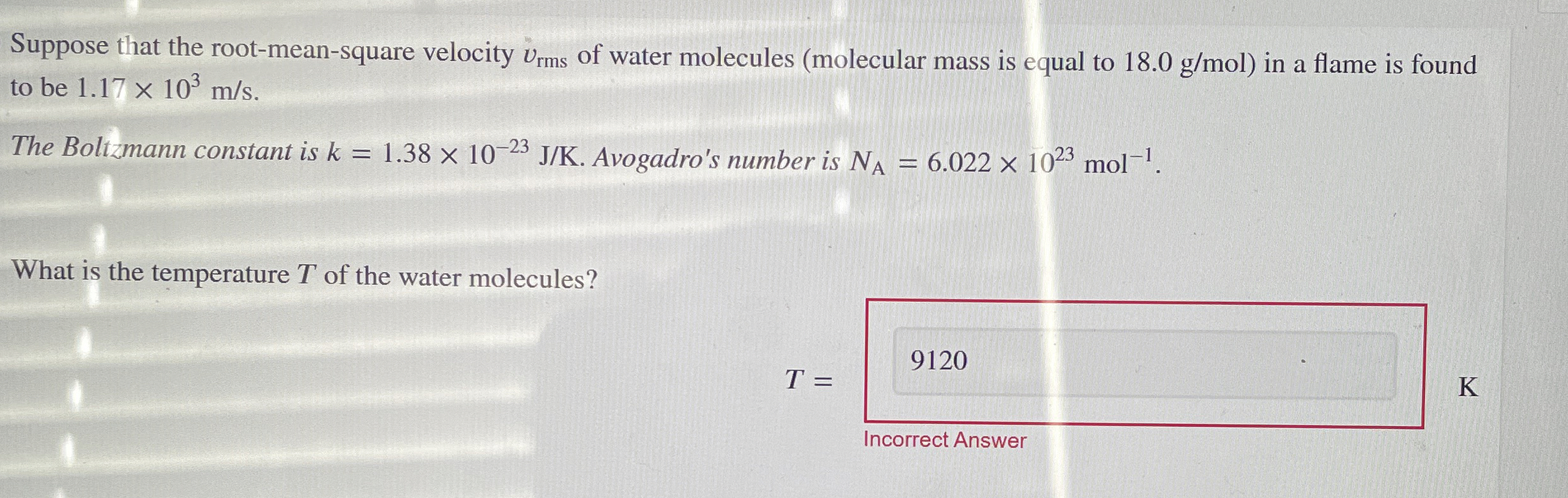
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


