Question: Suppose you are standardizing a sodium hydroxide solution with KHP (molar mass 204.2g/mol ) according to the equation KHP+NaOHH2O+NaKP You prepare the standard solution from
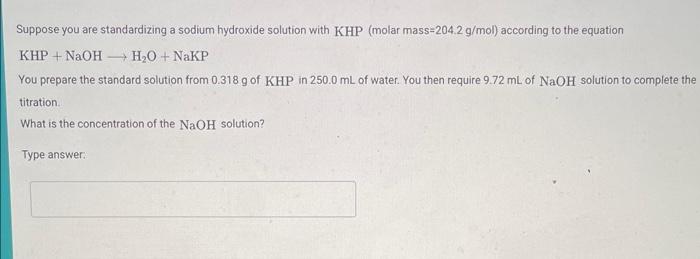
Suppose you are standardizing a sodium hydroxide solution with KHP (molar mass 204.2g/mol ) according to the equation KHP+NaOHH2O+NaKP You prepare the standard solution from 0.318g of KHP in 250.0mL of water. You then require 9.72mL of NaOH solution to complete the titration. What is the concentration of the NaOH solution? Type
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


