Question: Sustainable Design through process integration subject 5.3. Consider the coke oven gas (COG)-sweetening process shown in Fig. 5.32. The basic objective of COG sweetening is

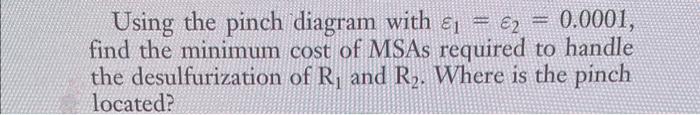
5.3. Consider the coke oven gas (COG)-sweetening process shown in Fig. 5.32. The basic objective of COG sweetening is the removal of acidic impurities, primarily hydrogen sulfide, from COG (a mixture of H2,CH4,CO,N2,NH3,CO2, and H2S ). Hydrogen sulfide is an undesirable impurity, because it is corrosive and contributes to SO2 emission when the COG is burnt. The existence of ammonia in COG and the selectivity of aqueous ammonia in absorbing H2S suggests that aqueous ammonia is a candidate solvent FIGURE 5-32 Sweetening of COG. Source: El-Halwagi and Manousiouthakis (1989). (process lean stream, S1 ). It is desirable that the ammonia recovered from the sour gas compensate for a large portion of the ammonia losses throughout the system and, thus, reduce the need for ammonia makeup. Besides ammonia, an external MSA (chilled methanol, S2 ) is also available for service to supplement the aqueous ammonia solution as needed. The purification of the COG involves washing the sour COG, R1, with sufficient aqueous ammonia and/or chilled methanol to absorb the required amounts of hydrogen sulfide. The acid gases are subsequently stripped from the solvents and the regenerated MSAs are recirculated. The stripped acid gases are fed to a "Claus unit" where elemental sulfur is recovered from hydrogen sulfide. In view of air pollution control regulations, the tail gases leaving the Claus unit, R2, should be treated for partial removal of the unconverted hydrogen sulfide. Table 5.10 summarizes the stream data. Using the pinch diagram with 1=2=0.0001, find the minimum cost of MSAs required to handle the desulfurization of R1 and R2. Where is the pinch located
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


