Question: T3T3. The process B -> C shown above is A. An isochoric process B. An isothermal process C. An isobaric process D. An adiabatic process
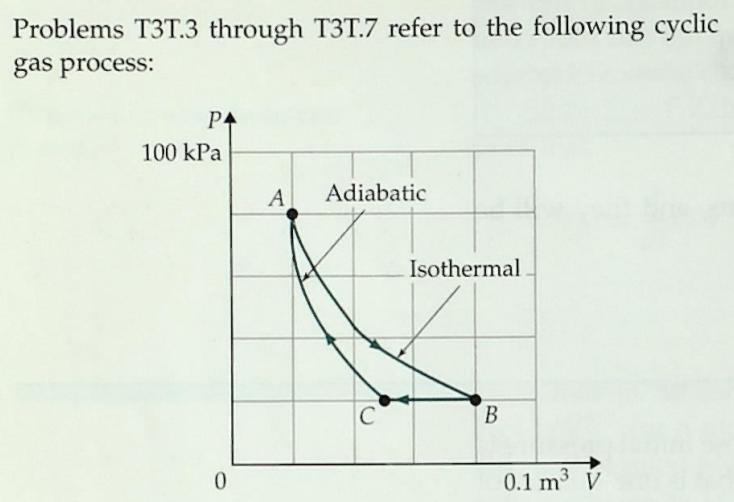
T3T3. The process B -> C shown above is
A. An isochoric process
B. An isothermal process
C. An isobaric process
D. An adiabatic process
E. An isometric process
F. None of the above
T3T4. Is the work energy flowing into or out of the gas in
process B -> C positive (A) or negative(B)?
Which of the values below is closest to the magnitude of W?
A. 0.6 J
B. 1.5 J
C. 300 J
D. 600 J
E. 1500 J
F. 3000 J
T3T5. What are the signs of Q, W, and delta U for the process
C -> A?
A. +, - , 0
B. 0, - , -
C. 0, + , +
D. + , + , 0
Problems T3T.3 through T3T.7 refer to the following cyclic gas process: P4 100 kPa A Adiabatic Isothermal C B 0.1 m V
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


