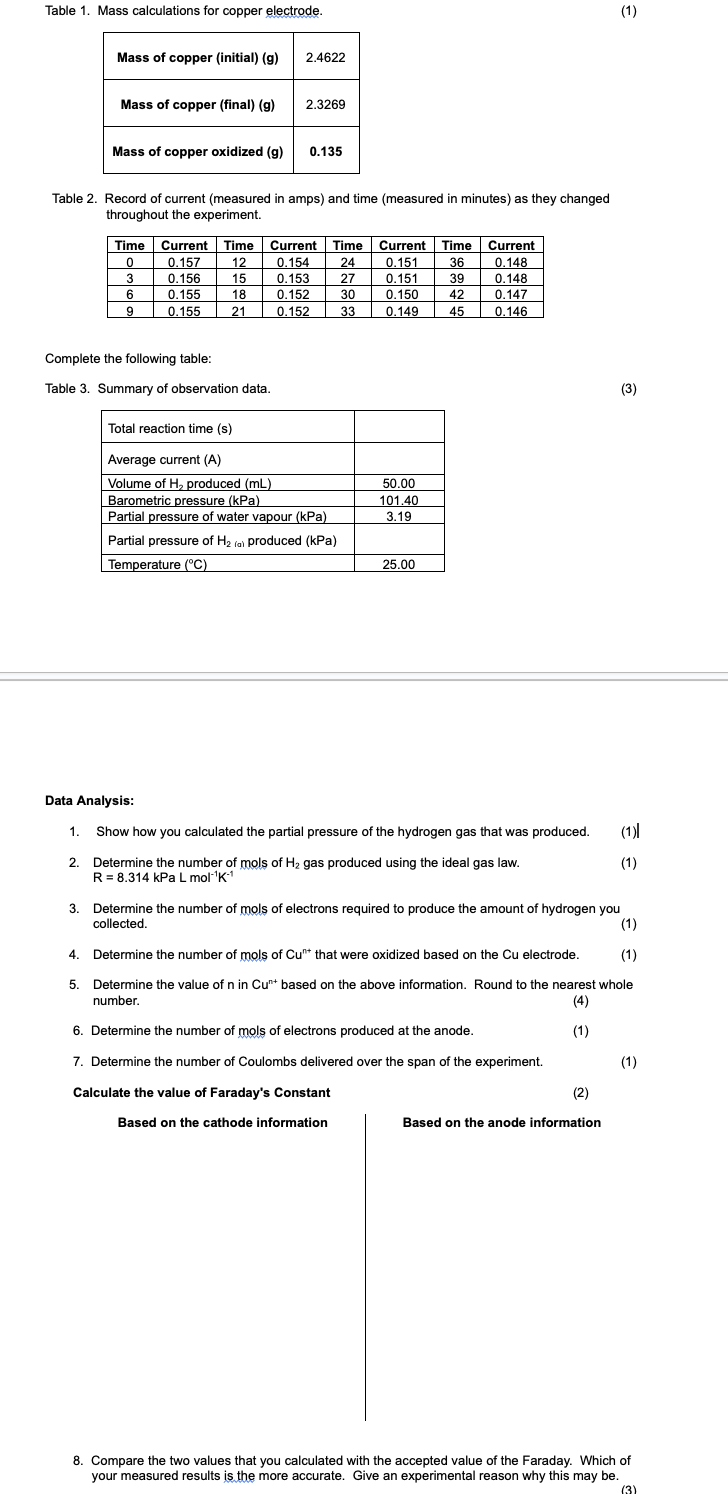
Question: Table 1 . Mass calculations for copper electrode. Table 2 . Record of current ( measured in amps ) and time ( measured in minutes
Table Mass calculations for copper electrode.
Table Record of current measured in amps and time measured in minutes as they changed
throughout the experiment.
Complete the following table:
Table Summary of observation data.
Data Analysis:
Show how you calculated the partial pressure of the hydrogen gas that was produced.
Determine the number of mols of gas produced using the ideal gas law.
Determine the number of mols of electrons required to produce the amount of hydrogen you
collected.
Determine the number of mols of that were oxidized based on the electrode.
Determine the value of in based on the above information. Round to the nearest whole
number.
Determine the number of mols of electrons produced at the anode.
Determine the number of Coulombs delivered over the span of the experiment.
Calculate the value of Faraday's Constant
Based on the cathode information
Based on the anode information
Compare the two values that you calculated with the accepted value of the Faraday. Which of
your measured results is the more accurate. Give an experimental reason why this may be
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


