Question: Table 2 Mixture Value of k (rate constant) 1 2 3 In the first part of the experiment the concentrations of the reactants changed in
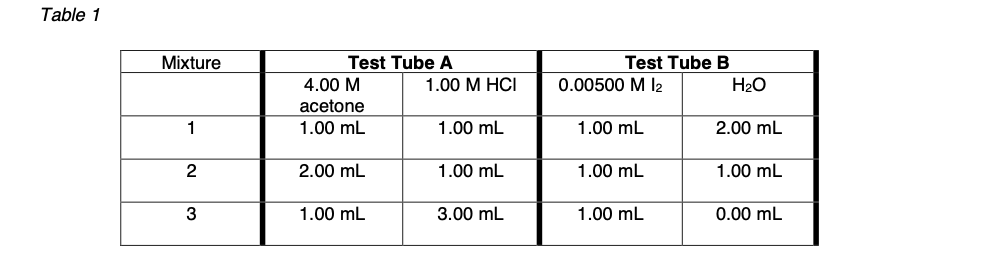
Table 2
| Mixture | Value of k (rate constant) |
|---|---|
| 1 | 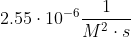 |
| 2 | 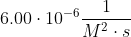 |
| 3 | 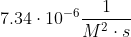 |
In the first part of the experiment the concentrations of the reactants changed in each trial (i.e. Table 1) despite the concentrations changing your experimental values of the rate constant (k) for each should remain similar. Do your (value of k) in Table 2 support this statement? (are the values of k similar to each other when comparing between mixture 1, 2 , 3? )
Please explain why or why not.
Table 1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


