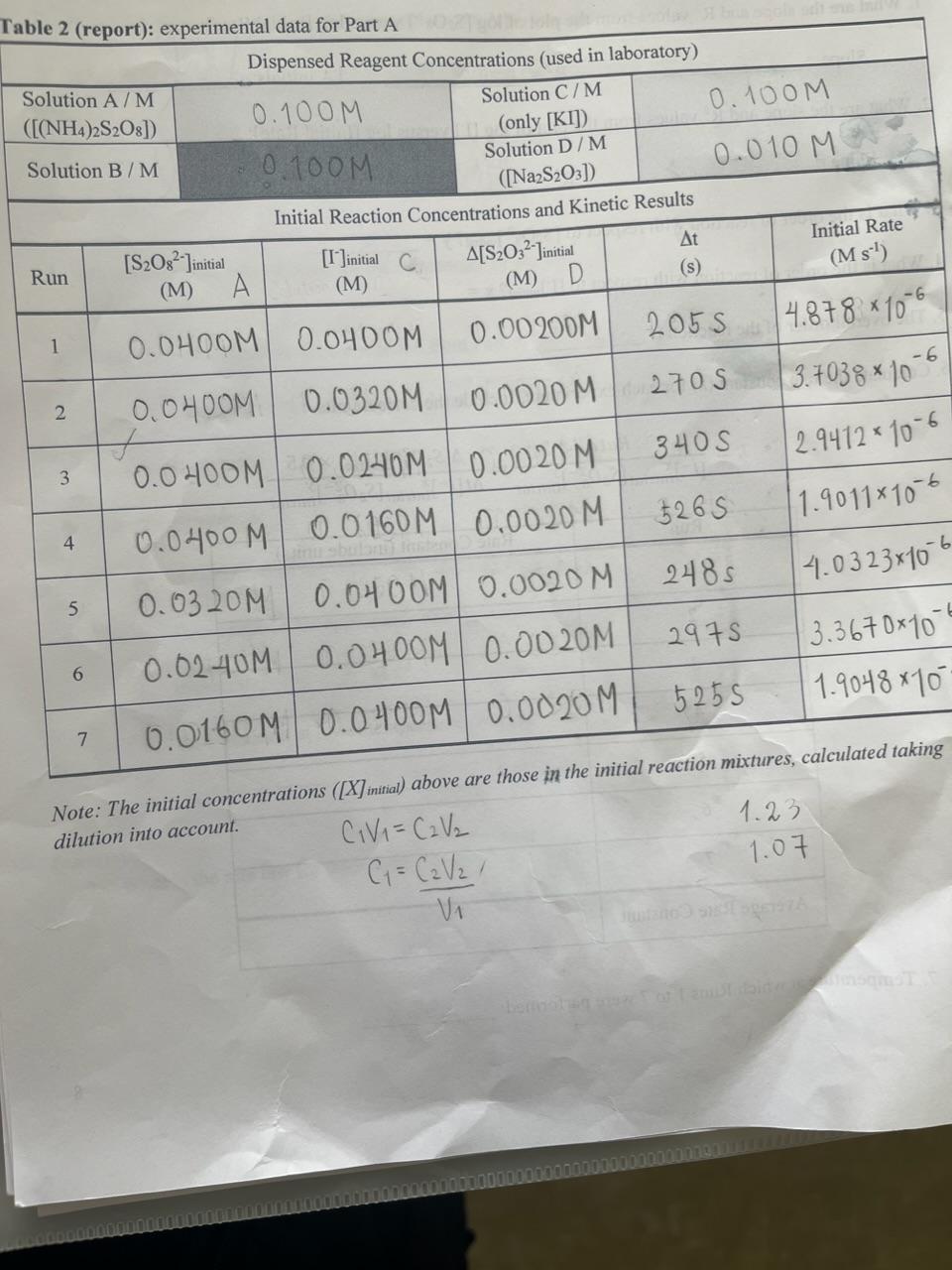
Question: Table 2 (rennrt): exnerimental data for Part A Note: The initial concentrations ([X]initial) above are those in the initial reaction muxtures, dilution into account. 3.
![concentrations ([X]initial) above are those in the initial reaction muxtures, dilution into](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f5d564a62_89266f8f5d4d5a80.jpg)
Table 2 (rennrt): exnerimental data for Part A Note: The initial concentrations ([X]initial) above are those in the initial reaction muxtures, dilution into account. 3. What is the order of reaction with respect to [S2O82]initial ? y= 4. What is the order of reaction with respect to [I]initial ? x= 5. The overall order of the reaction is 6. Calculate the rate constant (k) for each experiment. Include the correct units. k=[I]initialx[S2O82]initialyRate=t[I]initialx[S2O82]initialy[S2O32]initial0.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


