Question: Table 3: Determining Ksp* Average Temp (C) s(M)= [KNO3] (M) s= Ksp Density of HO at that Temp, (from CRC) Table 4: Determining AG from
![Table 3: Determining Ksp* Average Temp (C) s(M)= [KNO3] (M) s=](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d49397bf1_37966f8d4933d4af.jpg)
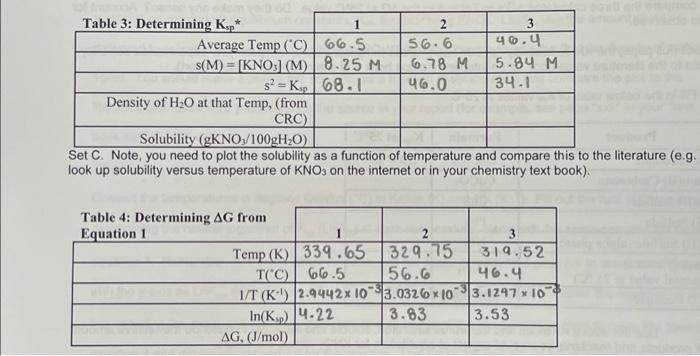
Set C. Note, you need to prot the solubility as a function of temperature and compare this to the literature (e.g. look up solubility versus temperature of KNO3 on the internet or in your chemistry text book). Set C. Note, you need to plot the solubility as a function of temperature and compare this to the literature (e.g look up solubility versus temperature of KNO3 on the internet or in your chemistry text book)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


