Question: Table (text book), calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and

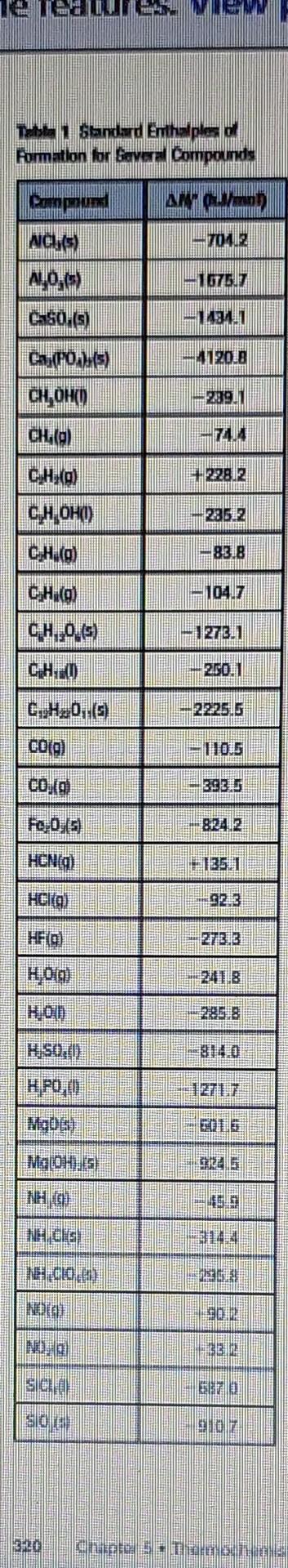
Table (text book), calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and water. 4NH3(g)+7O2(g)4NO2(g)+6H2O(l) Formation or Bowera Compounds
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


