Question: Table values you may need are here. Please give full solution with steps. Will upvote 6. (11 marks total) A well-insulated cylinder is fitted with
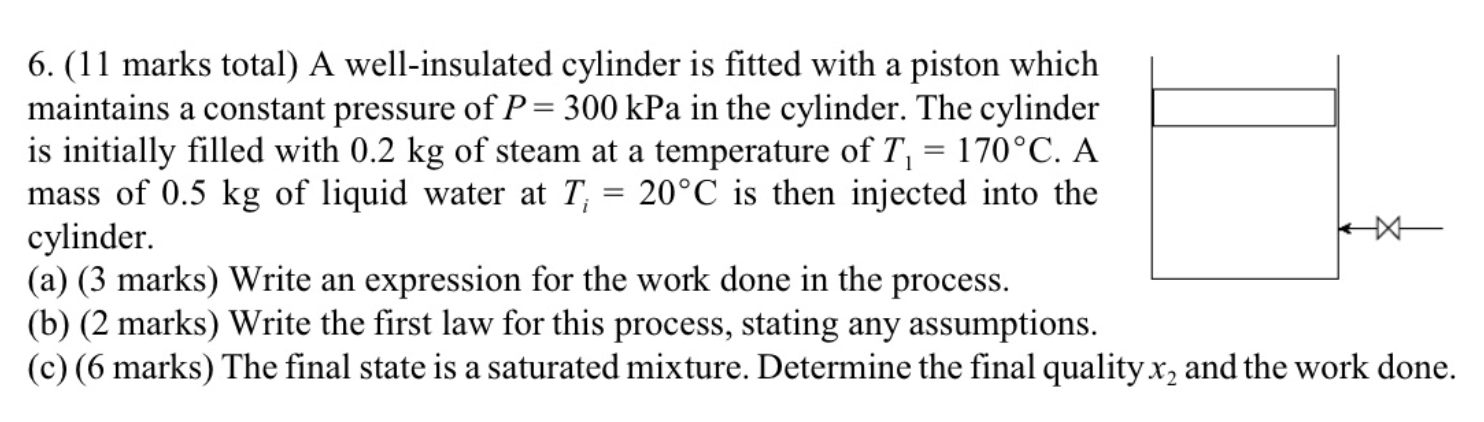
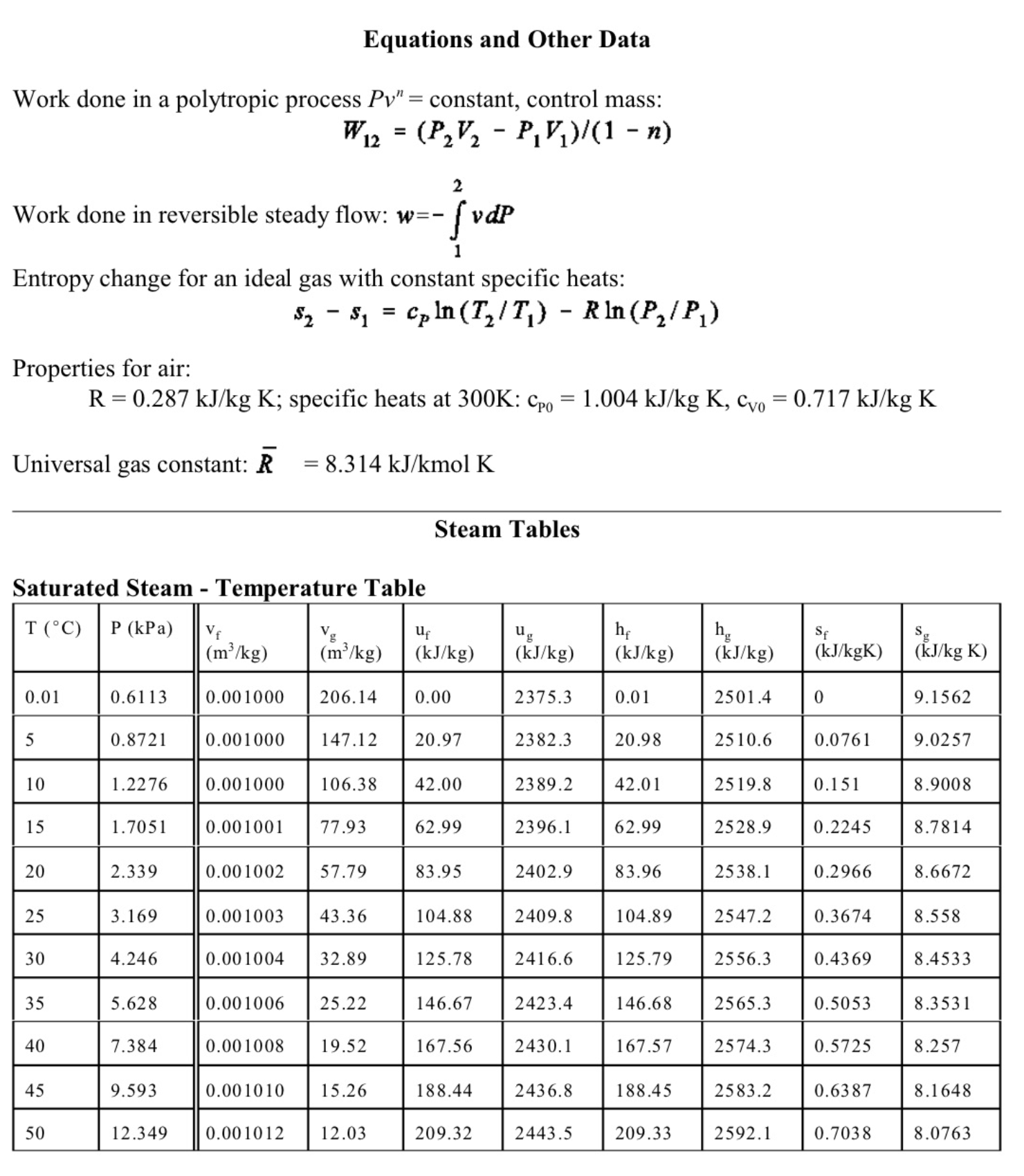
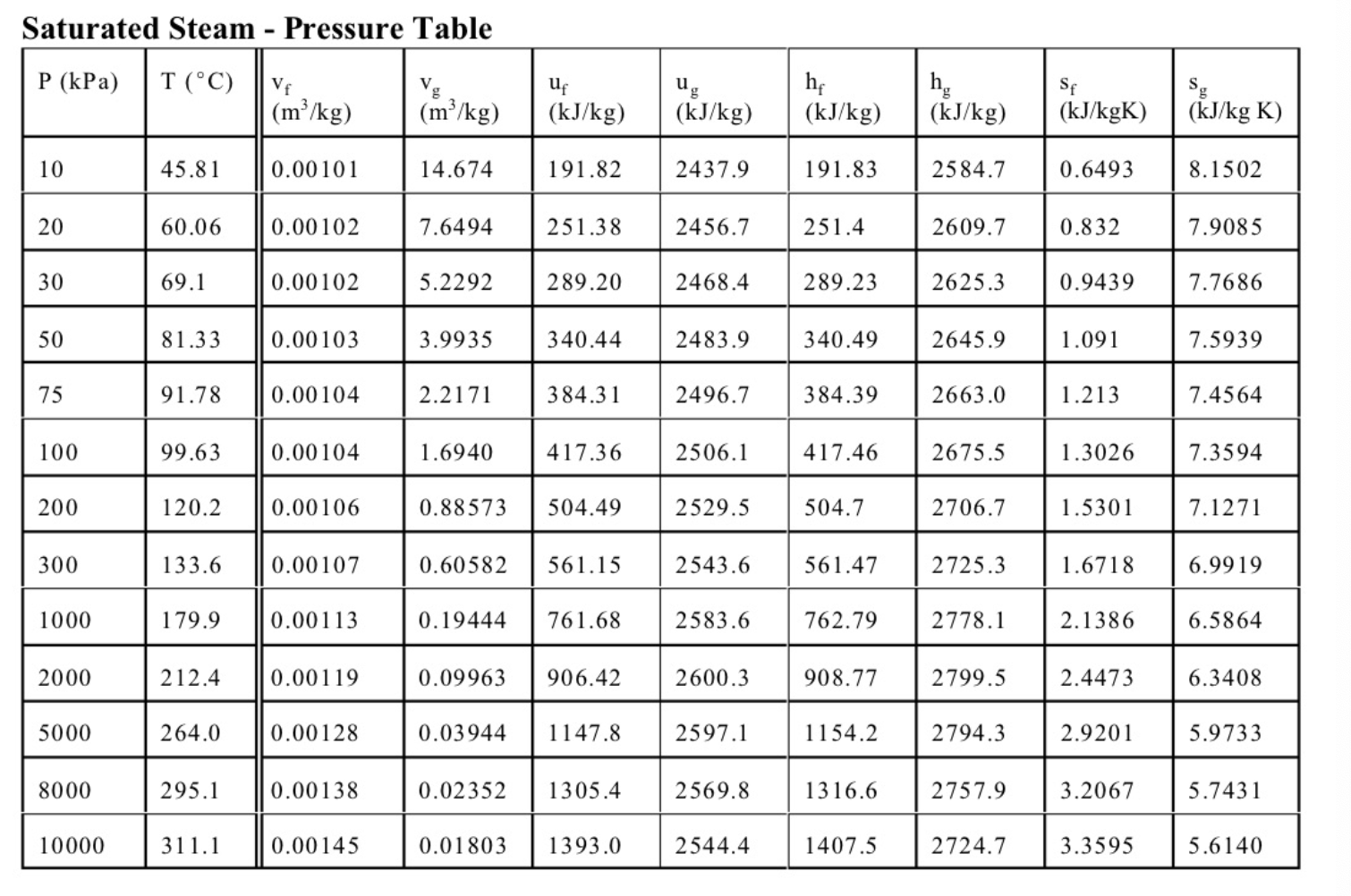
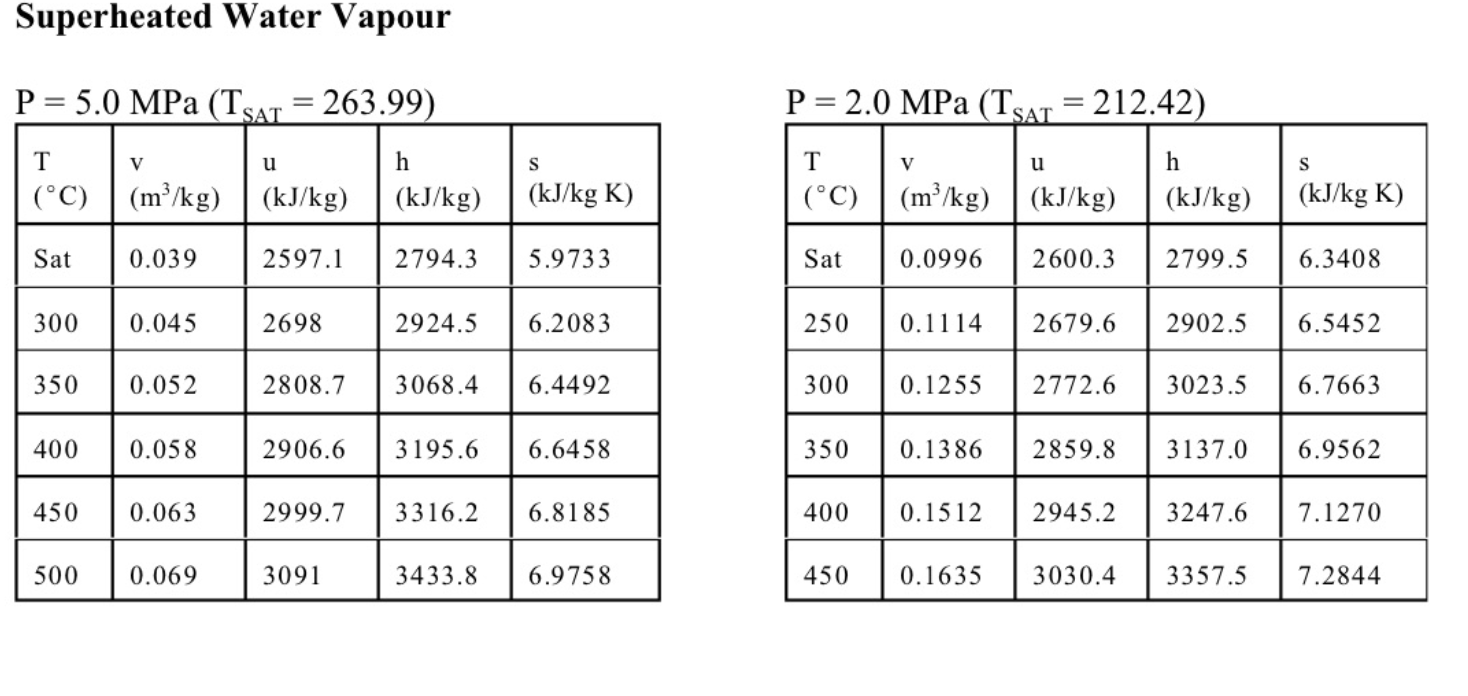
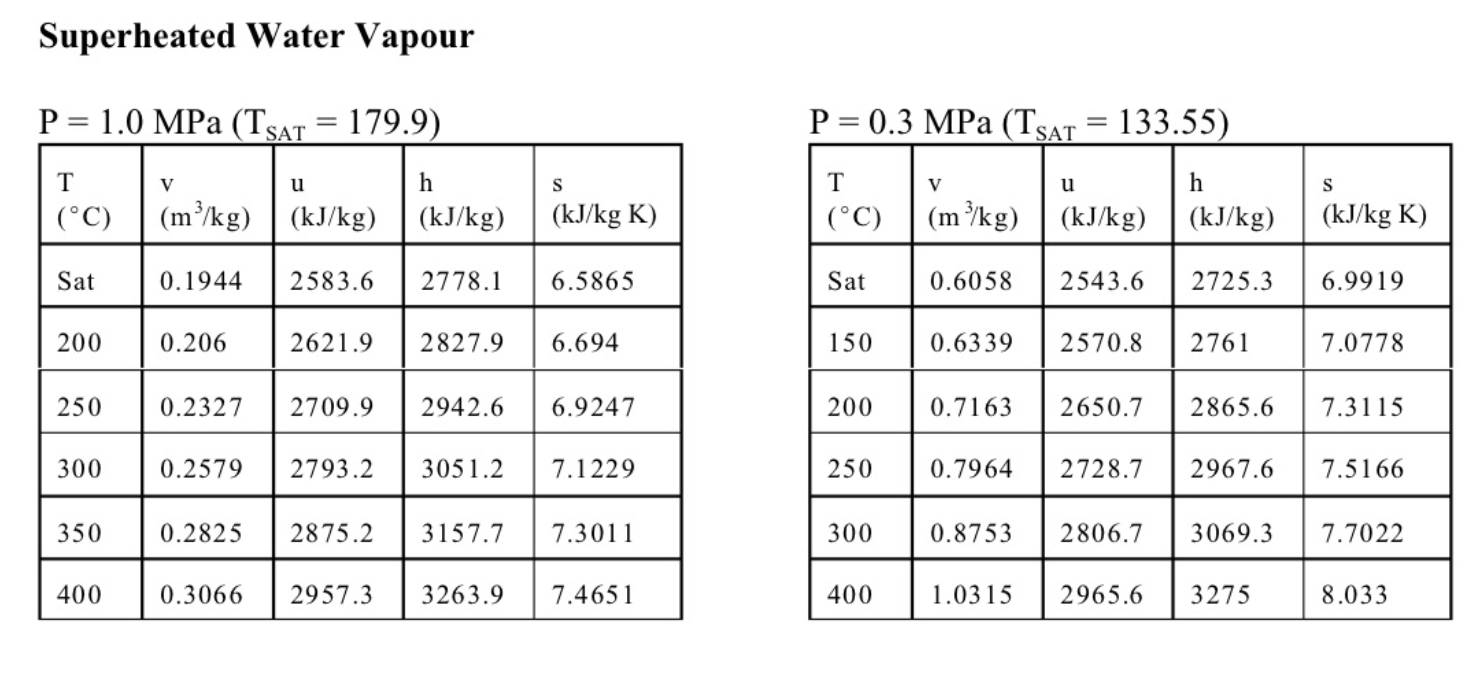
Table values you may need are here. Please give full solution with steps. Will upvote
6. (11 marks total) A well-insulated cylinder is fitted with a piston which maintains a constant pressure of P=300kPa in the cylinder. The cylinder is initially filled with 0.2kg of steam at a temperature of T1=170C. A mass of 0.5kg of liquid water at Ti=20C is then injected into the cylinder. (a) (3 marks) Write an expression for the work done in the process. (b) (2 marks) Write the first law for this process, stating any assumptions. (c) (6 marks) The final state is a saturated mixture. Determine the final quality x2 and the work done. Equations and Other Data Work done in a polytropic process Pvn= constant, control mass: W12=(P2V2P1V1)/(1n) Work done in reversible steady flow: w=12vdP Entropy change for an ideal gas with constant specific heats: s2s1=cPln(T2/T1)Rln(P2/P1) Properties for air: R=0.287kJ/kgK;specificheatsat300K:cP0=1.004kJ/kgK,cv0=0.717kJ/kgK Universal gas constant: R=8.314kJ/kmolK Steam Tables r 4 1. rr. Superheated Water Vapour P=5.0MPa(TCT=263.99) P=2.0MPa(TCAT=212.42) Superheated Water Vapour P=1.0MPa(TSAT=179.9) P=0.3MPa(TCT=133.55)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


