Question: Tabulate yield and relative hydrogen reactivities in the table shown. Relative yield comes from the GC area. Calculate the Reactivity/H by dividing the yield by
Tabulate yield and relative hydrogen reactivities in the table shown. Relative yield comes from the GC area. Calculate the Reactivity/H by dividing the yield by the number of hydrogens that give rise to that product. The relative reactivity is the normalized Reactivity/H; set the methyl group hydrogens of 1-chlorobutane (CH3CH2CH2CH2Cl) to 1.0 (corresponds to 1,4-dichlorobutane product). Statistical expectation assumes each hydrogen type has equal reactivity. Show an example calculation for each calculation type.
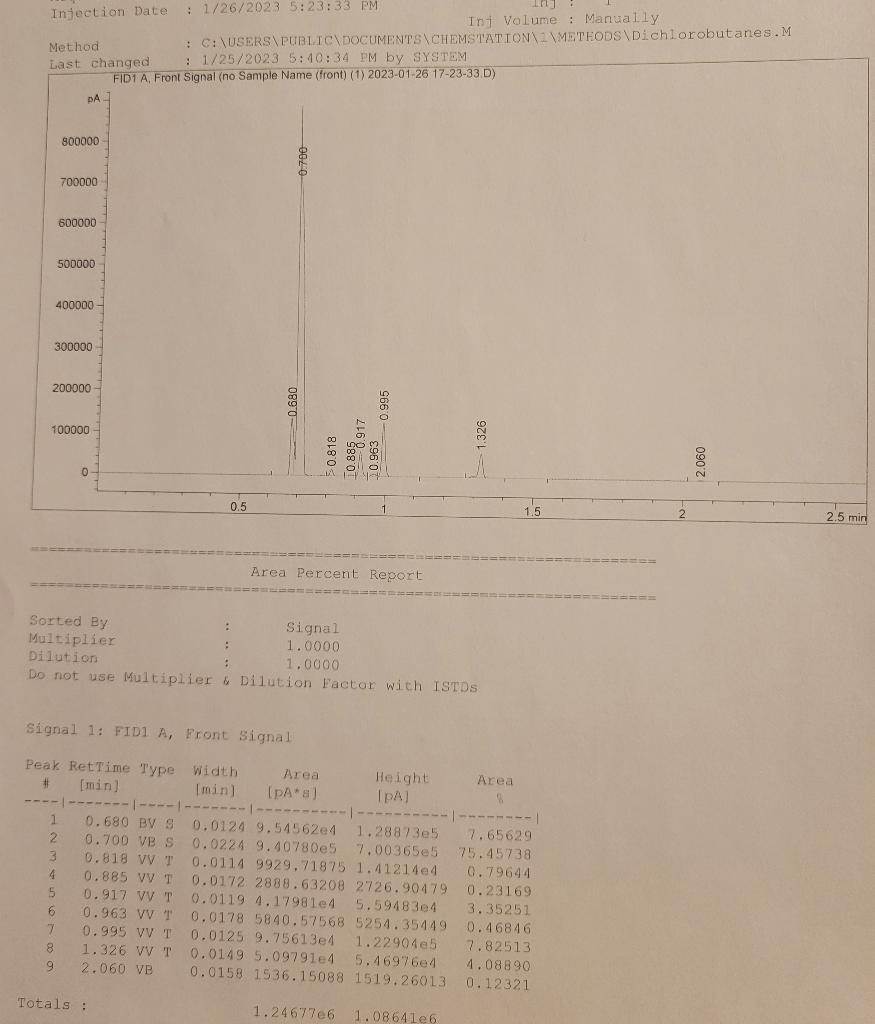
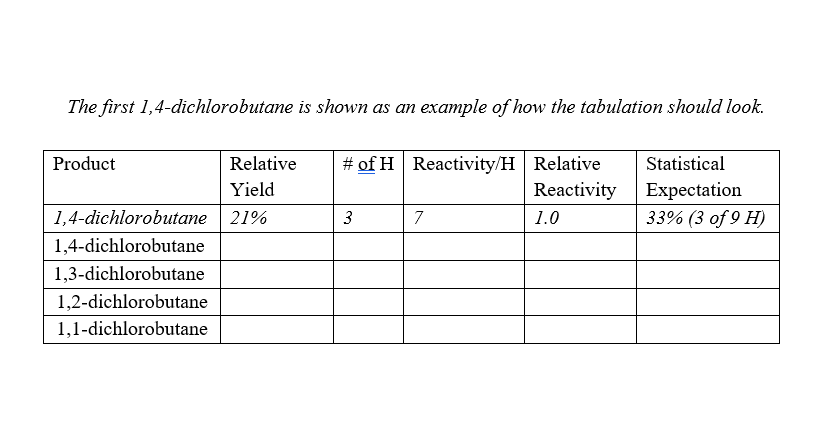
Injection Date : 1/26/2023 5:23:33 PM Inf Volume : Manually Method : C: \USERS PUBITC DOCUMENTS\CHEMSTATION \ZYMETHODS\DIchlorobutanes.M Last changed : 1/25/20235:40:34PM by SYSTEM Fin1 A Front Sianal (no Sample Name (front) (1) 2023-01-26 17-23-33.D) Area Percent Report Signal 1: FID1 A, Front Signal The first 1,4-dichlorobutane is shown as an example of how the tabulation should look. Injection Date : 1/26/2023 5:23:33 PM Inf Volume : Manually Method : C: \USERS PUBITC DOCUMENTS\CHEMSTATION \ZYMETHODS\DIchlorobutanes.M Last changed : 1/25/20235:40:34PM by SYSTEM Fin1 A Front Sianal (no Sample Name (front) (1) 2023-01-26 17-23-33.D) Area Percent Report Signal 1: FID1 A, Front Signal The first 1,4-dichlorobutane is shown as an example of how the tabulation should look
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


