Question: Take reference to this cell: ! Fe | FeSO4 (106 M) || H2SO4 (pH4) 02 | Fe (1) Use the following diagram to determine the
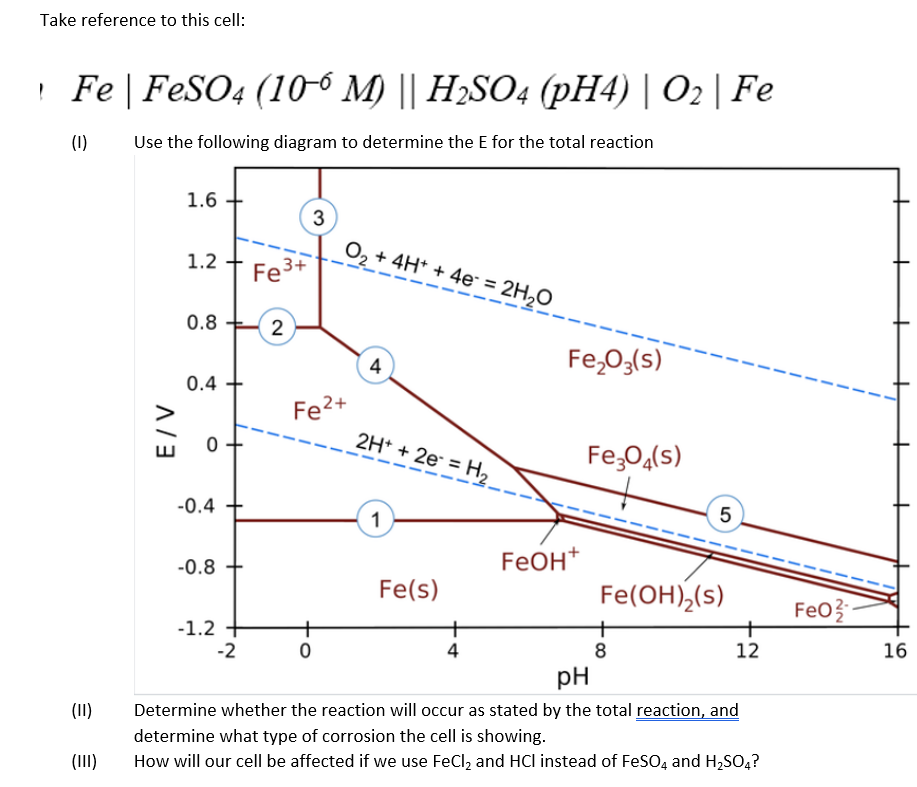
Take reference to this cell: ! Fe | FeSO4 (106 M) || H2SO4 (pH4) 02 | Fe (1) Use the following diagram to determine the E for the total reaction 1.6 3 0, + 4H+ + 4e = 2H2O 1.2 Fe 3+ 0.8 2 Fe 03(5) 4 0.4 Fe2+ E/V 0 2H+ + 2e = H2 Fe304(s) 5 FeO3 -0.4 1 -0.8 Feoht Fe(s) Fe(OH),(s) -1.2 + + + -2 0 4 8 12 pH Determine whether the reaction will occur as stated by the total reaction, and determine what type of corrosion the cell is showing. How will our cell be affected if we use FeCl2 and HCl instead of FeSO4 and H2SO4? 16 (11) (III)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


