Question: Task 1 fig. 9.7 (10th ed.) / Fig. 11.6 (9th ed.) / Shows the phase diagram of a copper (Cu) - silver (Ag) alloy. Look
Task 1 fig. 9.7 (10th ed.) / Fig. 11.6 (9th ed.) / Shows the phase diagram of a copper (Cu) - silver (Ag) alloy. Look at the alloy that contains 70 wt% Cu. Find the following at a 775 C. a)
(I)Relative mass of the and phases. (II) Relative mass of primary and eutectic mixture. (III) Relative mass of eutectic
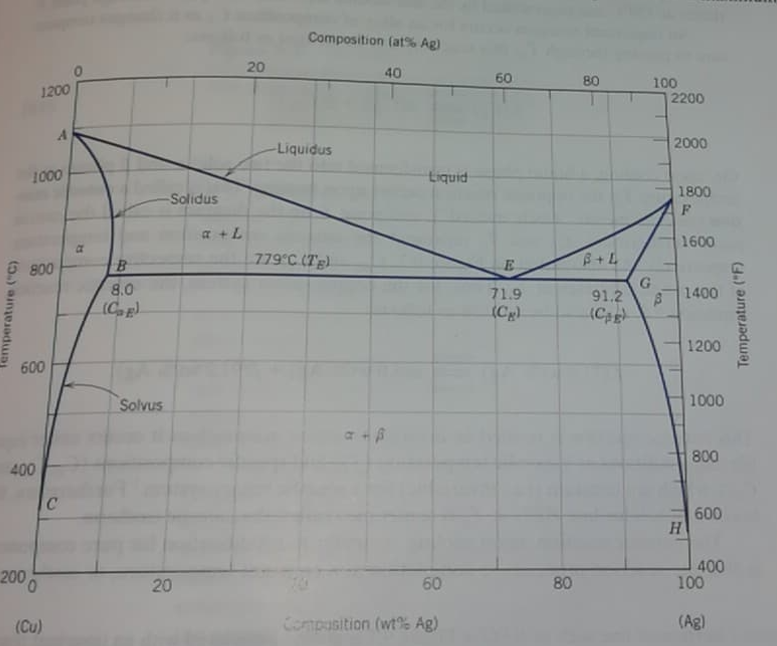
Composition (at AB) 20 40 60 80 1200 100 2200 -Liquidus 2000 1000 Liquid Solidus 1800 F 1600 a 779C (TE) 800 E B+ 4 B 8.0 (C G 1400 Temperature (C) 71.9 (Cp 8 91.2 Ce Temperature (F) 1200 600 Solvus 1000 800 400 600 H 2000 20 60 400 100 BO (Cu) Consition (wt% Ag) (Ag)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


