Question: Task 2 Aside from designing a new reactor, you are also planning to repurpose an unused CSTR (VCSTR = 4.3 m3) that has been placed
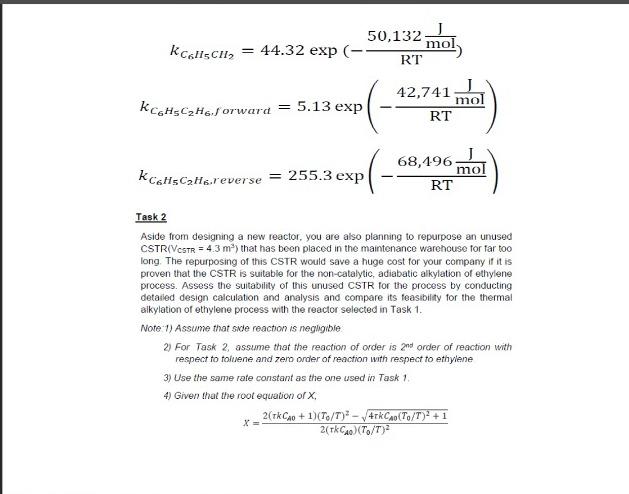
Task 2
Aside from designing a new reactor, you are also planning to repurpose an unused
CSTR (VCSTR = 4.3 m3) that has been placed in the maintenance warehouse for far too
long. The repurposing of this CSTR would save a huge cost for your company if it is
proven that the CSTR is suitable for the non-catalytic, adiabatic alkylation of ethylene
process. Assess the suitability of this unused CSTR for the process by conducting
detailed design calculation and analysis and compare its feasibility for the thermal
alkylation of ethylene process with the reactor selected in Task 1.
Note:1) Assume that side reaction is negligible.
2) For Task 2, assume that the reaction of order is 2nd order of reaction with
respect to toluene and zero order of reaction with respect to ethylene.
3) Use the same rate constant as the one used in Task 1.
4) Given that the root equation of X;
=
2(0 + 1)(0/)2 40(0/)2 + 1
2(0)(0/)2
kCallSCH = 50,132 mo 44.32 exp(- RT KCHECHS orward = 5.13 exp 42,741 mol RT k CssCaHa.reverse = 255.3 exp 68,496 RT mol Task 2 Aside from designing a new reactor, you are also planning to repurpose an unused CSTR(VCSTR = 4.3 m) that has been placed in the maintenance warehouse for far too long. The repurposing of this CSTR would save a huge cost for your company if it is proven that the CSTR is suitable for the non-catalytic, adiabatic alkylation of ethylene process. Assess the suitability of this unused CSTR for the process by conducting detailed design calculation and analysis and compare its feasibility for the thermal alkylation of ethylene process with the reactor selected in Task 1. Note 1) Assume that side reaction is negligible 2) For Task 2, assume that the reaction of order is 2nd order of reaction with respect to foluene and zero order of reaction with respect to ethylene 3) Use the same rate constant as the one used in Task 1 4) Given that the root equation of X, 2(TkC40 + 1)(To/T)2 - 4rk Cao(To/T)2 + 1 2(TCA)(To/T)2 kCallSCH = 50,132 mo 44.32 exp(- RT KCHECHS orward = 5.13 exp 42,741 mol RT k CssCaHa.reverse = 255.3 exp 68,496 RT mol Task 2 Aside from designing a new reactor, you are also planning to repurpose an unused CSTR(VCSTR = 4.3 m) that has been placed in the maintenance warehouse for far too long. The repurposing of this CSTR would save a huge cost for your company if it is proven that the CSTR is suitable for the non-catalytic, adiabatic alkylation of ethylene process. Assess the suitability of this unused CSTR for the process by conducting detailed design calculation and analysis and compare its feasibility for the thermal alkylation of ethylene process with the reactor selected in Task 1. Note 1) Assume that side reaction is negligible 2) For Task 2, assume that the reaction of order is 2nd order of reaction with respect to foluene and zero order of reaction with respect to ethylene 3) Use the same rate constant as the one used in Task 1 4) Given that the root equation of X, 2(TkC40 + 1)(To/T)2 - 4rk Cao(To/T)2 + 1 2(TCA)(To/T)2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


