Question: Temperature 4 C 21 C 30 C 37 C 90 C Absorbance at zero min Absorbance after 10 min of incubation A 0min A 10min


|
| Temperature | ||||
| 4 C | 21 C | 30 C | 37 C | 90 C | |
| Absorbance at zero min |
|
|
|
|
|
| Absorbance after 10 min of incubation |
|
|
|
|
|
| A0min A10min |
|
|
|
|
|
| Enzyme activity in 10 min (moles) |
|
|
|
|
|
| Enzyme activity (moles pyruvate/min) |
|
|
|
|
|
CALCULATIONS: show calculations
Insert figure
Figure x. Enzyme activity vs. temperature
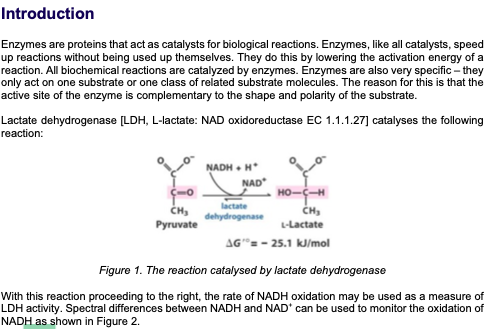
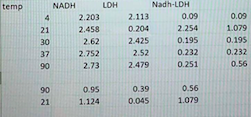
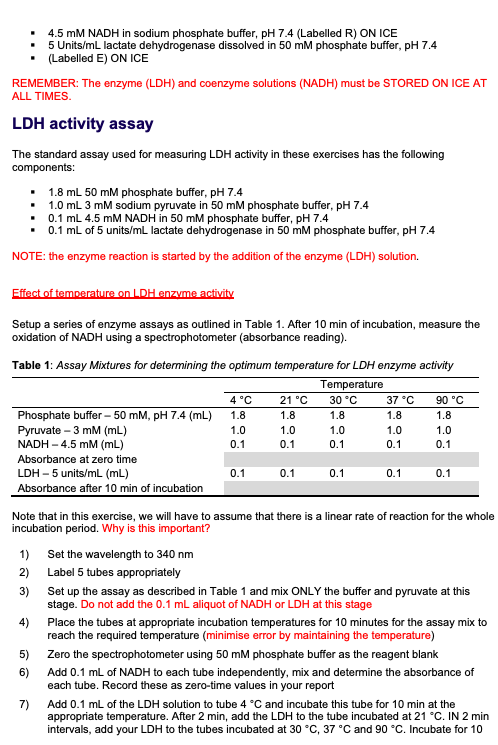
Introduction Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without being used up themselves. They do this by lowering the activation energy of a reaction. All biochemical reactions are catalyzed by enzymes. Enzymes are also very specific - they only act on one substrate or one class of related substrate molecules. The reason for this is that the active site of the enzyme is complementary to the shape and polarity of the substrate. Lactate dehydrogenase (LDH, L-lactate: NAD oxidoreductase EC 1.1.1.27] catalyses the following reaction: HO- NADH NAD (o CH lactate CH, dehydrogenase Pyruvate L-Lactate AG"=- 25.1 kJ/mol Figure 1. The reaction catalysed by lactate dehydrogenase With this reaction proceeding to the right, the rate of NADH oxidation may be used as a measure of LDH activity. Spectral differences between NADH and NAD can be used to monitor the oxidation of NADH as shown in Figure 2. 1.0 0.8 Oxidized (NAD) 0.6 Absorbance 0.4 Reduced (NADH) 0.2 0.0 220 240 260 280 300 320 340 360 380 Wavelength (nm) Figure 2. Absorption spectra of NAD and NADH The absorptivity (extinction coefficient) of NADH at 340 nm is: NADH = 6.22 x 10' L.mol-'cm', whilst ENAD+ 0 L.mol:".cm Therefore, the oxidation of NADH can be continuously monitored by measuring the decrease in absorbance at 340 nm. In this experiment, you will investigate two different parameters that affect enzyme activity: temperature and pH. You will use Beer's Law to calculate the rate of reaction A = EC Why do you need to use the extinction coefficient for calculations? To analyse the data you are collecting today, you will need to calculate initial velocity, V. This initial rate of reaction can be expressed simply as a change in absorbance per unit of time: for NADH consumption this would be AA340/min. This corresponds to the slope on your absorbance vs. time graph. For example, let's say that the straightest portion of your graph is between 0 minute and 1 minute, and the absorbance changes from 0.87 to 0.72 during this time. This means your slope, Vo, (0.87-072) is: V = V = 0.15/min (note that absorbance does not have any units) This is a simple way of calculation. However, it does not allow us to compare the enzyme activity between different reaction conditions. For example, some other enzyme might show the same vo Therefore, it is more useful to express the rate in terms of the actual amount of NADH consumed per unit time; this allows researchers working under different experimental conditions to compare their results. Hence, in the current practical, you will calculate the Vas nanomoles per minute (nmol/min) or micromoles per minute (pmol/min). This can be achieved by using the Beer-Lambert law. Make sure you understand this as in the future this can be applied to a wide range of scenarios. We will cover some of this aspect in Lecture 12 - Applications of Biological Chemistry. Reagents 50 mM sodium phosphate buffer, pH 7.4 3 mM sodium pyruvate dissolved in 50 mM phosphate buffer, pH 7.4 temp NADH LOH 4 2.203 21 2.458 2.62 2.752 90 2.73 Nadh-LDH 2.113 0.09 0.204 2.254 2.425 0.195 2.52 0.232 2.479 0.251 30 37 0.09 1.079 0.195 0.232 0.56 90 0.95 1.124 0.39 0.045 0.56 1.079 21 4.5 mM NADH in sodium phosphate buffer, pH 7.4 (Labelled R) ON ICE . 5 Units/mL lactate dehydrogenase dissolved in 50 mM phosphate buffer, pH 7.4 . (Labelled E) ON ICE REMEMBER: The enzyme (LDH) and coenzyme solutions (NADH) must be STORED ON ICE AT ALL TIMES. LDH activity assay The standard assay used for measuring LDH activity in these exercises has the following components: 1.8 ml 50 mM phosphate buffer, pH 7.4 1.0 mL 3 mM sodium pyruvate in 50 mm phosphate buffer, pH 7.4 . 0.1 mL 4.5 mM NADH in 50 mM phosphate buffer, pH 7.4 . 0.1 mL of 5 units/mL lactate dehydrogenase in 50 mM phosphate buffer, pH 7.4 NOTE: the enzyme reaction is started by the addition of the enzyme (LDH) solution. Effect of temperature on LDH enzyme activity Setup a series of enzyme assays as outlined in Table 1. After 10 min of incubation, measure the oxidation of NADH using a spectrophotometer (absorbance reading). Table 1: Assay Mixtures for determining the optimum temperature for LDH enzyme activity Temperature 4C 21 C 30 C 37 C 90 C Phosphate buffer - 50 mM, pH 7.4 (mL) 1.8 1.8 Pyruvate - 3 mM (ML) 1.0 1.0 1.0 1.0 1.0 NADH - 4.5 mm (mL) 0.1 0.1 0.1 0.1 0.1 Absorbance at zero time LDH - 5 units/mL (mL) 0.1 0.1 0.1 0.1 Absorbance after 10 min of incubation 1.8 1.8 1.8 0.1 Note that in this exercise, we will have to assume that there is a linear rate of reaction for the whole incubation period. Why is this important? 1) Set the wavelength to 340 nm 2) Label 5 tubes appropriately 3) Set up the assay as described in Table 1 and mix ONLY the buffer and pyruvate at this stage. Do not add the 0.1 mL aliquot of NADH or LDH at this stage 4) Place the tubes at appropriate incubation temperatures for 10 minutes for the assay mix to reach the required temperature (minimise error by maintaining the temperature) 5) Zero the spectrophotometer using 50 mM phosphate buffer as the reagent blank 6) Add 0.1 mL of NADH to each tube independently, mix and determine the absorbance of each tube. Record these as zero-time values in your report 7) Add 0.1 mL of the LDH solution to tube 4 C and incubate this tube for 10 min at the appropriate temperature. After 2 min, add the LDH to the tube incubated at 21 C. IN 2 min intervals, add your LDH to the tubes incubated at 30 C, 37 C and 90 C. Incubate for 10 min at the respective temperatures (this is just so that it is easier to read the absorbance values - This step is not compulsory) 8) After 10 minutes of incubation, determine the absorbance of the tubes and record the values in your report. (If you follow the 2-minute interval in the previous step then read the absorbance of the 4C assay at 10 minutes, read the absorbance of the 21C assay at 12 minutes etc.) 9) Using the extinction coefficient provided, determine the amount of NADH (in umoles) consumed in each assay 10) Assuming that there is a 1:1 stoichiometry between NADH and the substrate being consumed, determine the rate of reaction (umoles of substrate consumed per minute) for each temperature 11) Plot enzyme activity vs. temperature 12) Determine the optimum temperature for LDH Effect of pH on LDH enzyme activity
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


